PHYSICAL
SCIENCES
Grade 11
TERM 1
RESOURCE
PACK
Gr11_Physical_Science_Term1_Resource_Book.indb 1 2018/12/24 8:21:35 AM

Contents
Worksheets 3
Topic 1: Vectors in Two Dimensions 4
Topic 2: Newton’s Laws and Application of Newton’s Laws 13
Topic 3: Atomic Combinations (Molecular Structure) 33
Topic 4: Intermolecular Forces and Chemistry of Water 48
Formal Experiment 59
Technical Instructions 61
Newton’s Second Law of Motion 64
Marking Guidelines 74
Assessments 81
Topic 1: Vectors in Two Dimensions 82
Topic 2: Newton’s Laws and Application of Newton’s Laws 89
Topic 3: Atomic Combinations (Molecular Structure) 101
Topic 4: Intermolecular Forces and Chemistry of Water 113
Gr11_Physical_Science_Term1_Resource_Book.indb 2 2018/12/24 8:21:35 AM
WORKSHEETS
Gr11_Physical_Science_Term1_Resource_Book.indb 3 2018/12/24 8:21:35 AM

RESOURCE PACK
4
Topic 1 – Vectors in Two Dimensions
WORKSHEET
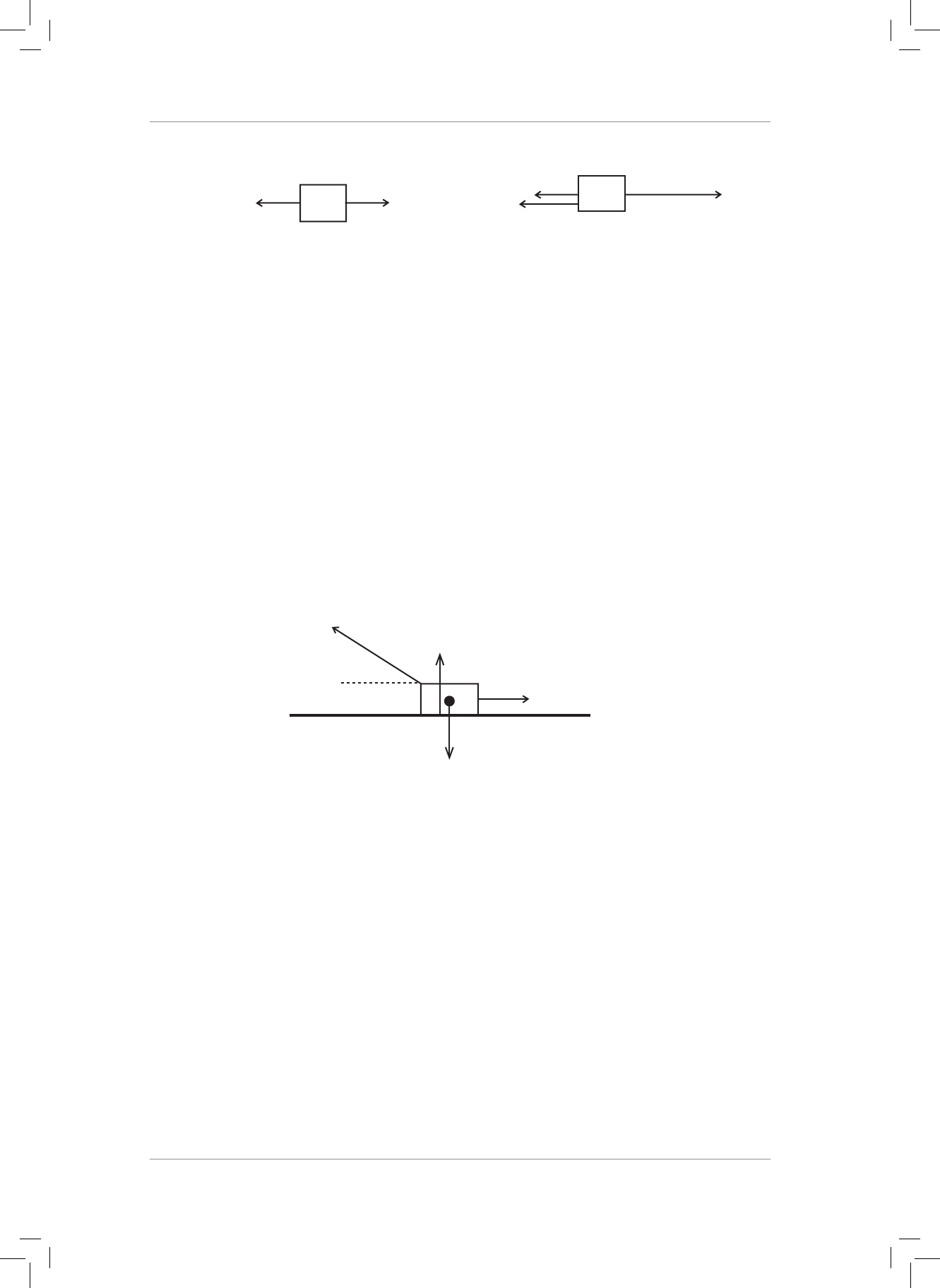
1. A forward horizontal force of 50 N is applied to a crate. A second horizontal force of
180 N is applied to the crate in the opposite direction. Determine the magnitude and
direction of the resultant force acting on the crate. (2)
2. e athlete at point A runs 150 m east, then 70 m west and then 100 m east. Determine
the resultant displacement of the athlete relative to point A. (2)
3. ree forces act on an object in the vertical plane. Two forces of 500 N and 300 N act
vertically upwards and the third force of 600 N acts vertically downwards. Determine
the resultant force acting on the object. (2)
4. An aircra ies 8 km north from an airport and then 12 km east.
4.1 Use the tail-to-head method to draw a neat labelled displacement vector diagram.
Draw in the resultant displacement vector. (3)
4.2 Determine the magnitude and direction of the resultant displacement of the aircra
relative to the airport. (5)
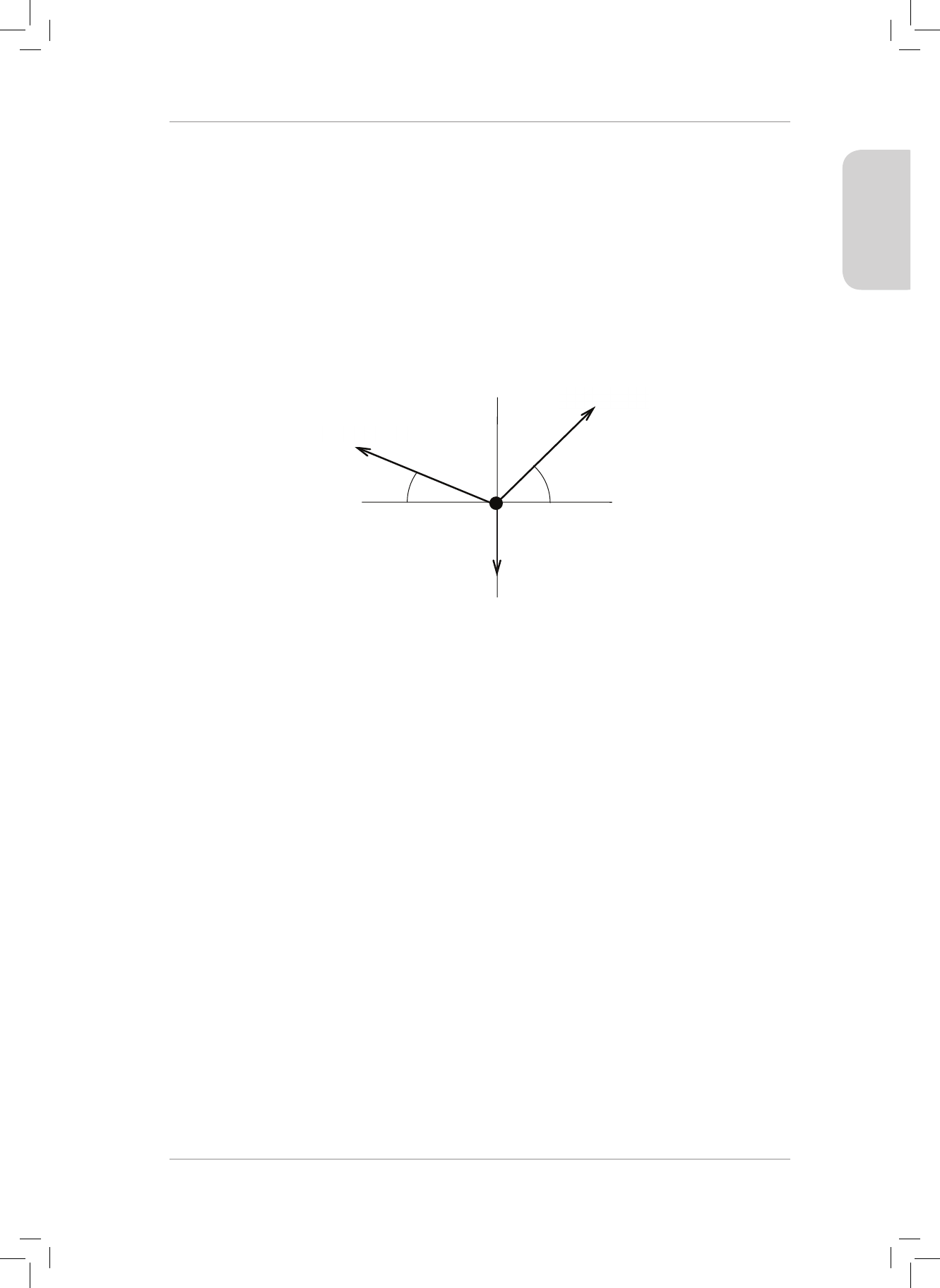
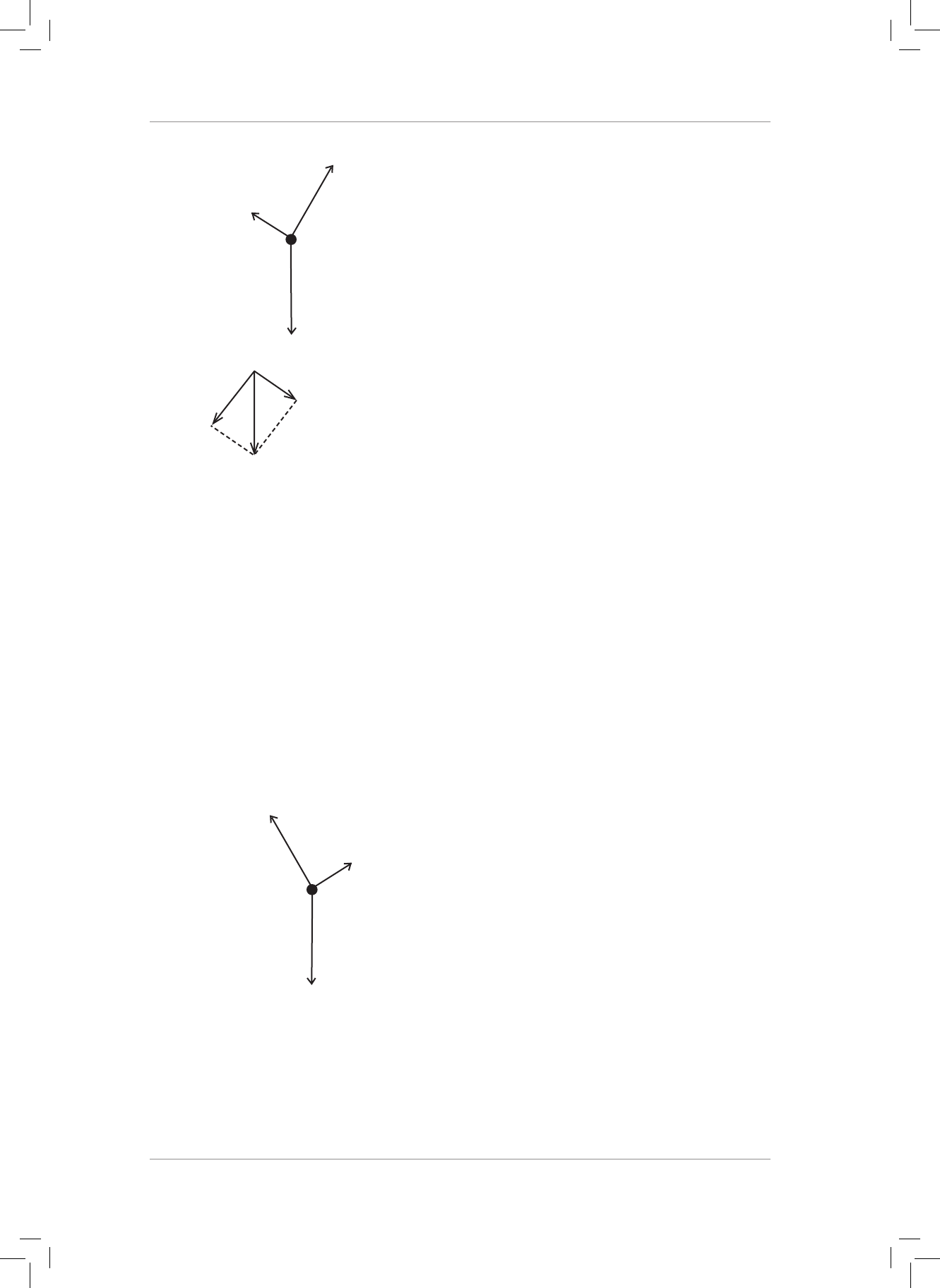
5. Draw the following force vectors (not necessarily to scale) in the Cartesian plane acting
from the origin:
5.1 F
1
= 20 N acts to the right in the horizontal plane.
5.2 F
2
= 50 N acts upwards in the vertical plane.
5.3 F
3
= 80 N acts to the le in the horizontal plane.
5.4 F
4
= 90 N acts downwards in the vertical plane. (4)
6. Consider the four forces in question 5.
6.1 Determine the resultant horizontal force and the resultant vertical force. (4)
6.2 Use the tail-to-tail method to calculate the magnitude and direction of the resultant
of the two forces in Question 6.1. Draw a neat labelled vector diagram to support
your calculation. (7)
6.3 Now use the tail-to-head method to draw a neat labelled vector diagram. (3)
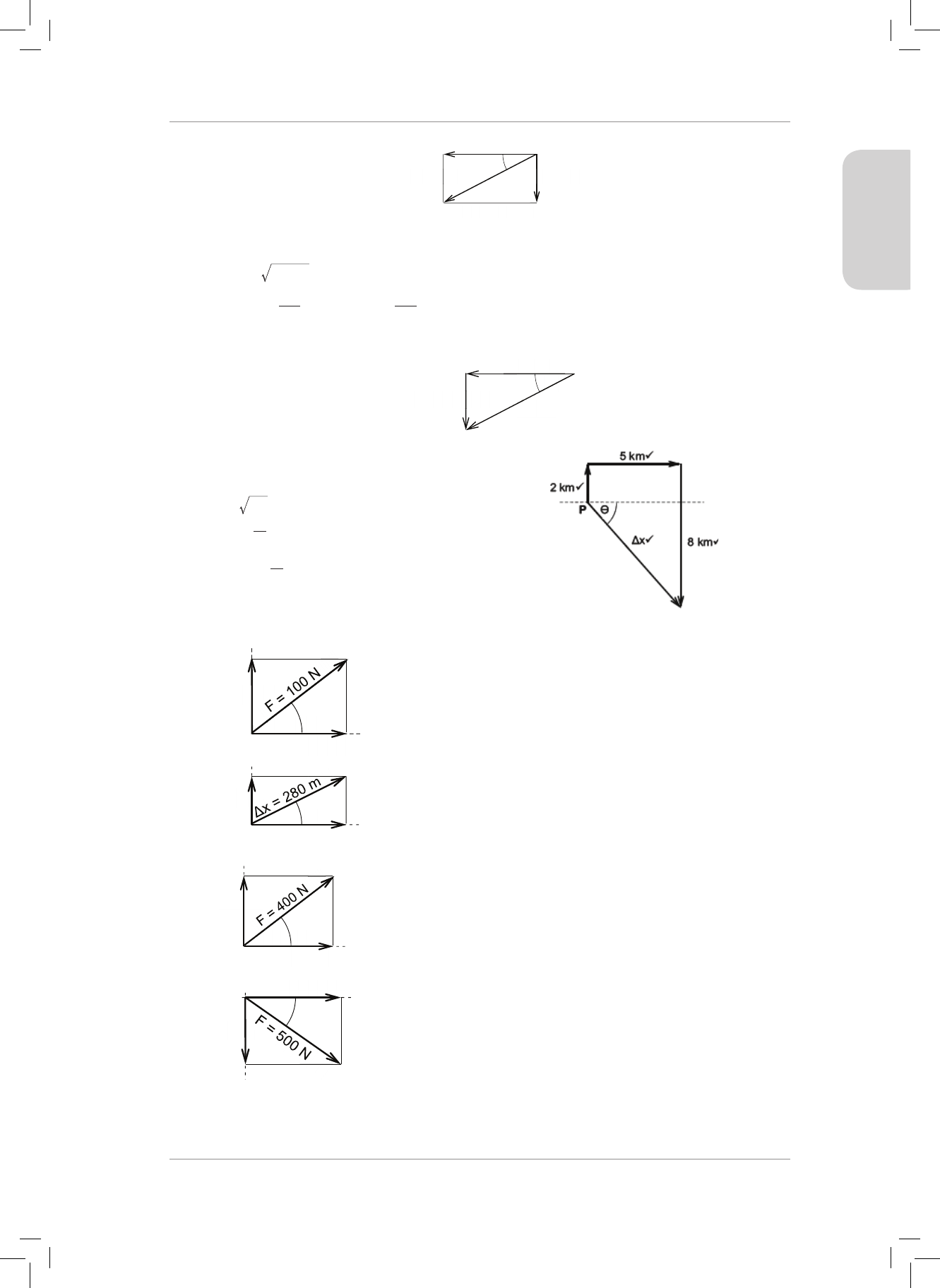
7. A drone at point P is own 2 km north, then 5 km east and then 8 km south. Find the
magnitude and direction of the resultant displacement of the drone from point P. Draw
a neat labelled displacement vector diagram to support your calculation. (8)
Gr11_Physical_Science_Term1_Resource_Book.indb 4 2018/12/24 8:21:35 AM
5
WORKSHEETS
8. Resolve each of the following vectors into their perpendicular components. In each case
draw a neat, labelled vector diagram (not necessarily to scale).
8.1 A 100 N force which acts at 30° above the horizontal. (6)
8.2 A displacement of 280 m on a bearing of 070°. (6)
8.3 A 400 N force which acts at 50° to the vertical. (6)
8.4 A 500 N force which acts at 40° below the horizontal. (6)
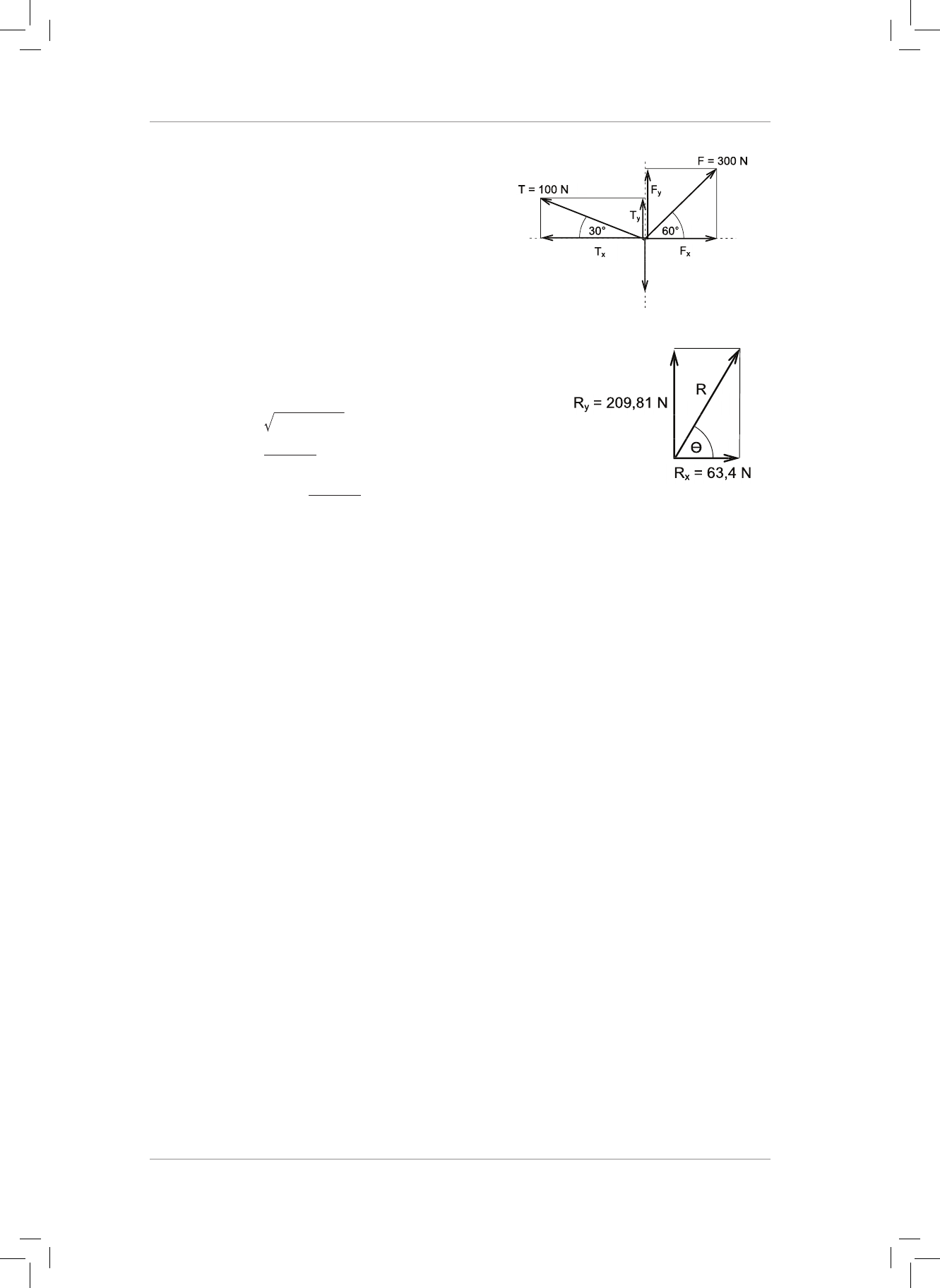
9. ree forces T, F and W act on an object as shown in the diagram below.
3
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors in two dimensions
9. Three forces T, F and W act on an object as shown in the diagram below.
F = 300 N
W = 100 N
T = 100 N
60° 30°
9.1 Determine the perpendicular components of force F. (6)
9.2 Determine the perpendicular components of force T (6)
9.3 Determine the magnitude and direction of the resultant force acting on the object.
Draw a neat labelled vector diagram to support your calculation. (8)
9.1 Determine the perpendicular components of force F. (6)
9.2 Determine the perpendicular components of force T. (6)
9.3 Determine the magnitude and direction of the resultant force acting on the object.
Draw a neat labelled vector diagram to support your calculation. (8)
Gr11_Physical_Science_Term1_Resource_Book.indb 5 2018/12/24 8:21:36 AM
RESOURCE PACK
6
CONSOLIDATION EXERCISE
TOTAL: 40 MARKS
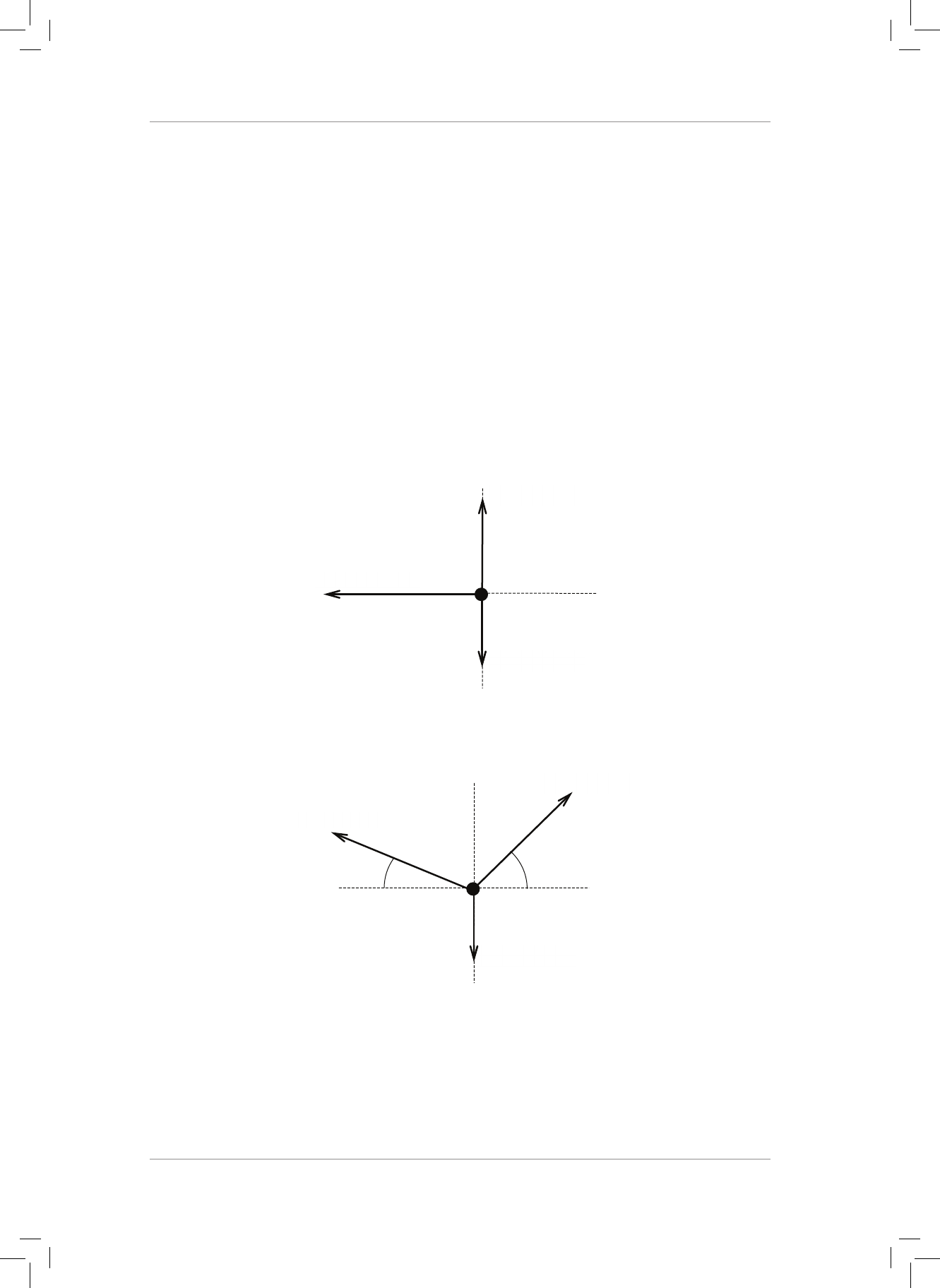
1. Dene a resultant vector. (2)
2. A block is pulled by two horizontal forces of 15 N and 25 N to the le, and by two
horizontal forces of 10 N and 30 N to the right.
2.1 Draw these four force vectors in the Cartesian plane (acting at the origin). (2)
2.2 Find the magnitude and direction of the resultant force. (2)
3. A learner hikes 8 km north and 12 km west. Using the tail-to-head method, determine
the magnitude and direction of the learner’s resultant displacement. (5)
4. ree forces, A, B and C act on an object as shown in the diagram. Determine the
magnitude and direction of the resultant force acting on the object.
4
Grade 11 PHYSICAL SCIENCES Term 1
TOPIC 2: Newton’s Laws and Application of Newton’s Laws
Topic 1: Vectors In Two Dimensions
GRADE 11: CONSOLIDATION QUESTIONS TOTAL: 40 MARKS
1. Dene a resultant vector. (2)
2. A block is pulled by two horizontal forces of 15 N and 25 N to the left, and by two
horizontal forces of 10 N and 30 N to the right.
2.1 Draw these four force vectors in the Cartesian plane (acting at the origin). (2)
2.2 Find the magnitude and direction of the resultant force. (2)
3. A student hikes 8 km North and the 12 km West. Using the tail-to-head method,
determine the magnitude and direction of the student’s resultant displacement. (5)
4. Three forces, A, B and C act on an object as shown in the diagram. Determine the
magnitude and direction of the resultant force acting on the object.
B = 40 N
C = 10 N
A = 60 N
(6)
5. Consider the three forces acting on an object as shown below.
T = 500 N
W = 200 N
F = 300 N
45°
20°
5.1 Calculate the horizontal and vertical components of force T. (4)
5.2 Calculate the horizontal and vertical components of force F. (4)
5.3 Determine the magnitude and direction of the resultant horizontal force. (2)
5.4 Determine the magnitude and direction of the resultant vertical force. (2)
5.5 Using the tail-to-tail method, draw a neat labelled force diagram and determine
the magnitude and direction of the resultant force acting on the object. (5)
(6)
5. Consider the three forces acting on an object as shown in the diagram below.
4
Grade 11 PHYSICAL SCIENCES Term 1
TOPIC 2: Newton’s Laws and Application of Newton’s Laws
Topic 1: Vectors In Two Dimensions
GRADE 11: CONSOLIDATION QUESTIONS TOTAL: 40 MARKS
1. Dene a resultant vector. (2)
2. A block is pulled by two horizontal forces of 15 N and 25 N to the left, and by two
horizontal forces of 10 N and 30 N to the right.
2.1 Draw these four force vectors in the Cartesian plane (acting at the origin). (2)
2.2 Find the magnitude and direction of the resultant force. (2)
3. A student hikes 8 km North and the 12 km West. Using the tail-to-head method,
determine the magnitude and direction of the student’s resultant displacement. (5)
4. Three forces, A, B and C act on an object as shown in the diagram. Determine the
magnitude and direction of the resultant force acting on the object.
B = 40 N
C = 10 N
A = 60 N
(6)
5. Consider the three forces acting on an object as shown below.
T = 500 N
W = 200 N
F = 300 N
45° 20°
5.1 Calculate the horizontal and vertical components of force T. (4)
5.2 Calculate the horizontal and vertical components of force F. (4)
5.3 Determine the magnitude and direction of the resultant horizontal force. (2)
5.4 Determine the magnitude and direction of the resultant vertical force. (2)
5.5 Using the tail-to-tail method, draw a neat labelled force diagram and determine
the magnitude and direction of the resultant force acting on the object. (5)
5.1 Calculate the horizontal and vertical components of force T. (4)
5.2 Calculate the horizontal and vertical components of force F. (4)
5.3 Determine the magnitude and direction of the resultant horizontal force. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 6 2018/12/24 8:21:36 AM
7
WORKSHEETS
5.4 Determine the magnitude and direction of the resultant vertical force. (2)
5.5 Using the tail-to-tail method, draw a neat labelled force diagram and determine the
magnitude and direction of the resultant force acting on the object. (5)
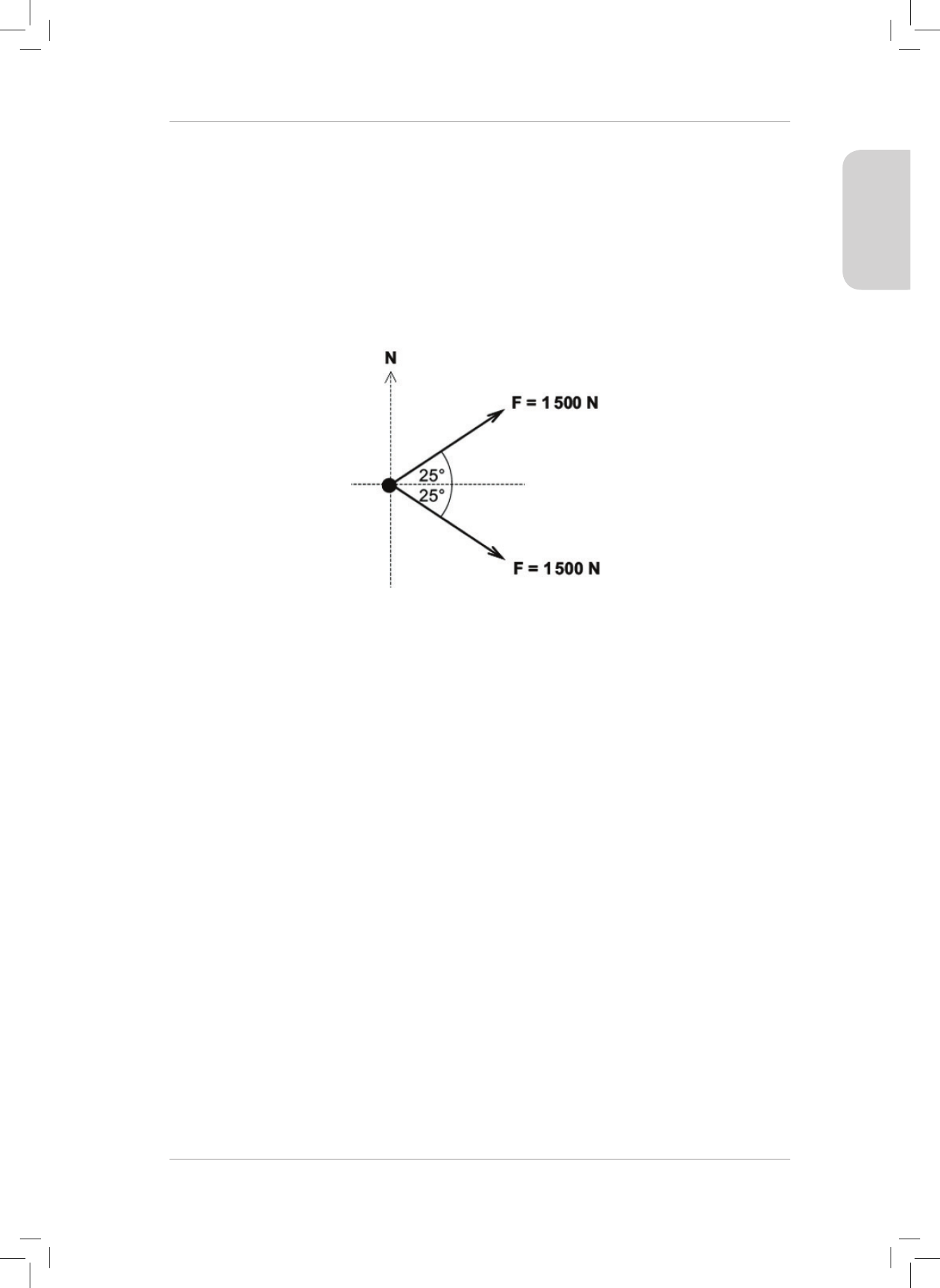
6. e diagram below shows a top view of a tug boat that is pulled by two forces. North is
shown.
Use the component method to nd the magnitude and direction of the resultant force
acting on the tugboat. State the direction as a bearing, clockwise from north.
PHYSICAL SCIENCES RESOURCE PACK
GRADE 11
TERM 1
WORKSHEETS
PAGE 7 DIAGRAM FOR QUESTION 6.
PAGE 8 QUESTION 4.2
x
2
= 8
2
+ 12
2
x =
208 = 14,42 km
tan θ =
θ = tan
-1
= 56,31
o
x = 14,42 km on a bearing of 056,31
o
PAGE 9 DIAGRAM FOR QUESTION 7
(6)
[40]
Gr11_Physical_Science_Term1_Resource_Book.indb 7 2018/12/24 8:21:37 AM
RESOURCE PACK
8
MARKING GUIDELINES
1. Choose forward as the positive (+) direction.
en:
NR 50 180 130 130
=+ +- =- =
^h
backwards (2)
2. Choose east as the positive (+) direction.
en:
mx 150 70 100 180T
=+ +- +=
^h
east (2)
3. Choose upwards as the positive (+) direction.
en:
NR 500 300 600 200 200
=+++-=+=
^h
upwards (2)
4. 4.1
6
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: WORKSHEET MEMORANDUM
1. Choose forward as the positive (+) direction.
Then:
RN50 180 130 130
=+ +- =- =
^h
backwards (2)
2.
Choose east as the positive (+) direction.
Then:
()xm150 70 100 180
D
=+ +- +=
East (2)
3. Choose upwards as the positive (+) direction.
Then:
RN
500 300 600 200 200
=+ ++-=+=
^h
upwards (2)
4.1
Ɵ
8 km
12 km
Δx
(3)
4.2
,
() ,
,,
°
°
tantan
x
xk
m
xkmbearing
812
208 14 42
8
12
8
12
56 31
14 42 56 31
222
1
=+
==
== =
=
-
(5)
5.
(4)
6.1
RN20 80 60 60
x
=+ +- =- =
^h
left
RN50 90 40 40
y
=+ +- =- =
^h
downwards
(4)
6.2
40 N
60 N
Ɵ
R
40 N
60 N
R 60 40
222
=+
,RN5200 72 11
==
() , °tantan
60
40
60
40
33 69
1
i
== =
-
,,°RNat belowthe horizontal72 11 33 69
=
(7)
F
3
= 80 N
F
1
= 20
F
2
= 50 N
F
4
= 90 N
(3)
4.2
,
,
,,
tan
tan
km
km on abearing of
x
x
x
812
208 14 42
8
12
8
12
56 31
14 42 056 31
22 2
1
c
c
{{
{
{
{
i
i
=+
==
=
==
=
-
bl
(5)
5.
6
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: WORKSHEET MEMORANDUM
1. Choose forward as the positive (+) direction.
Then:
RN50 180 130 130
=+ +- =- =
^h
backwards (2)
2.
Choose east as the positive (+) direction.
Then:
()xm150 70 100 180
D
=+ +- +=
East (2)
3. Choose upwards as the positive (+) direction.
Then:
RN
500 300 600 200 200
=+ ++-=+=
^h
upwards (2)
4.1
Ɵ
8 km
12 km
Δx
(3)
4.2
,
() ,
,,
°
°
tantan
x
xk
m
xkmbearing
812
208 14 42
8
12
8
12
56 31
14 42 56 31
222
1
=+
==
== =
=
-
(5)
5.
(4)
6.1
RN20 80 60 60
x
=+ +- =- =
^h
left
RN50 90 40 40
y
=+ +- =- =
^h
downwards
(4)
6.2
40 N
60 N
Ɵ
R
40 N
60 N
R 60 40
222
=+
,RN5200 72 11
==
() , °tantan
60
40
60
40
33 69
1
i
== =
-
,,°RNat belowthe horizontal72 11 33 69
=
(7)
F
3
= 80 N
F
1
= 20
F
2
= 50 N
F
4
= 90 N
6
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: WORKSHEET MEMORANDUM
1. Choose forward as the positive (+) direction.
Then:
RN50 180 130 130
=+ +- =- =
^h
backwards (2)
2.
Choose east as the positive (+) direction.
Then:
()xm150 70 100 180
D
=+ +- +=
East (2)
3. Choose upwards as the positive (+) direction.
Then:
RN
500 300 600 200 200
=+ ++-=+=
^h
upwards (2)
4.1
Ɵ
8 km
12 km
Δx
(3)
4.2
,
() ,
,,
°
°
tantan
x
xk
m
xkmbearing
812
208 14 42
8
12
8
12
56 31
14 42 56 31
222
1
=+
==
== =
=
-
(5)
5.
(4)
6.1
RN20 80 60 60
x
=+ +- =- =
^h
left
RN50 90 40 40
y
=+ +- =- =
^h
downwards
(4)
6.2
40 N
60 N
Ɵ
R
40 N
60 N
R 60 40
222
=+
,RN5200 72 11
==
() , °tantan
60
40
60
40
33 69
1
i
== =
-
,,°RNat belowthe horizontal72 11 33 69
=
(7)
F
3
= 80 N
F
1
= 20
F
2
= 50 N
F
4
= 90 N
(4)
6. 6.1
NleftR 20 80 60 60
x
{
=+ +- =- =
^h
NdownwardsR 50 90 40 40
y
{
=+ +- =- =
^h
(4)
Gr11_Physical_Science_Term1_Resource_Book.indb 8 2018/12/24 8:21:42 AM
9
PHYSICAL SCIENCES RESOURCE PACK
GRADE 11
TERM 1
WORKSHEETS
PAGE 7 DIAGRAM FOR QUESTION 6.
PAGE 8 QUESTION 4.2
x
2
= 8
2
+ 12
2
x =
208 = 14,42 km
tan θ =
θ = tan
-1
= 56,31
o
x = 14,42 km on a bearing of 056,31
o
PAGE 9 DIAGRAM FOR QUESTION 7
6.3
(3)
7
x 56
22
2
=+
,xkm61 781
D
==
() , °tantan
5
6
5
6
50 19
1
i
== =
-
,,xkmbearing781 140 19
=
c
(8)
8.1
.(). ,
°°
coscosFF N30 100 30 86 60
x
== =
right
.().
°°
si
ns
inFF N30 100 30 50
y
== =
upwards
vector diagram
(6)
8.2
.().°°coscosxx20 280 20
D
==
= 263,11m east
.().°°sinsinyx20 280 20
D
==
= 95,77 m north
vector diagram
(6)
8.3
.().°°coscosFF 40 400 40
x
==
= 306,42N right
.().°°sinsinFF40 400 40
y
==
= 257,11N upwards
vector diagram
(6)
8.4
.().°°coscosFF 40 500 40
x
==
= 383,02N right
.().°°sinsinFF40 500 40
y
==
= 321,39N
downwards
vector diagram
(6)
9.1
(). °cosF 300 60
x
=
= 150N right
().
°
sinF 300 60
y
=
=259,81N up
(6)
9.2
(). °cosT 100 30
x
=
=86,60 N left
(). °sinT 100 30
y
=
=50N up
(6)
7
Grade 11 PHYSICAL SCIENCES Term 1
2 km
8 km
5 km
P
Δx
P
Ɵ
30°
F
x
F
y
20°
x
y
40°
F
x
F
y
40°
F
x
F
y
Ɵ
R
40 N
60 N
F = 300 N
W = 100 N
T = 100 N
60°
30°
F
x
F
y
T
x
T
y
Topic 1: Vectors In Two Dimensions
6.3
(3)
7
x 56
22
2
=+
,xkm61 781
D
==
() , °tantan
5
6
5
6
50 19
1
i
== =
-
,,xkmbearing781 140 19
=
c
(8)
8.1
.(). ,
°°
coscosFF N30 100 30 86 60
x
== =
right
.().
°°
si
ns
inFF N30 100 30 50
y
== =
upwards
vector diagram
(6)
8.2
.().°°coscosxx20 280 20
D
==
= 263,11m east
.().°°sinsinyx20 280 20
D
==
= 95,77 m north
vector diagram
(6)
8.3
.().°°coscosFF 40 400 40
x
==
= 306,42N right
.().°°sinsinFF40 400 40
y
==
= 257,11N upwards
vector diagram
(6)
8.4
.().°°coscosFF 40 500 40
x
==
= 383,02N right
.().°°sinsinFF40 500 40
y
==
= 321,39N
downwards
vector diagram
(6)
9.1
(). °cosF 300 60
x
=
= 150N right
().
°
sinF 300 60
y
=
=259,81N up
(6)
9.2
(). °cosT 100 30
x
=
=86,60 N left
(). °sinT 100 30
y
=
=50N up
(6)
7
Grade 11 PHYSICAL SCIENCES Term 1
2 km
8 km
5 km
P
Δx
P
Ɵ
30°
F
x
F
y
20°
x
y
40°
F
x
F
y
40°
F
x
F
y
Ɵ
R
40 N
60 N
F = 300 N
W = 100 N
T = 100 N
60°
30°
F
x
F
y
T
x
T
y
Topic 1: Vectors In Two Dimensions
6.3
(3)
7
x 56
22
2
=+
,xkm61 781
D
==
() , °tantan
5
6
5
6
50 19
1
i
== =
-
,,xkmbearing781 140 19
=
c
(8)
8.1
.(). ,
°°
coscosFF N30 100 30 86 60
x
== =
right
.().
°°
si
ns
inFF N30 100 30 50
y
== =
upwards
vector diagram
(6)
8.2
.().°°coscosxx20 280 20
D
==
= 263,11m east
.().°°sinsinyx20 280 20
D
==
= 95,77 m north
vector diagram
(6)
8.3
.().°°coscosFF 40 400 40
x
==
= 306,42N right
.().°°sinsinFF40 400 40
y
==
= 257,11N upwards
vector diagram
(6)
8.4
.().°°coscosFF 40 500 40
x
==
= 383,02N right
.().°°sinsinFF40 500 40
y
==
= 321,39N
downwards
vector diagram
(6)
9.1
(). °cosF 300 60
x
=
= 150N right
().
°
sinF 300 60
y
=
=259,81N up
(6)
9.2
(). °cosT 100 30
x
=
=86,60 N left
(). °sinT 100 30
y
=
=50N up
(6)
7
Grade 11 PHYSICAL SCIENCES Term 1
2 km
8 km
5 km
P
Δx
P
Ɵ
30°
F
x
F
y
20°
x
y
40°
F
x
F
y
40°
F
x
F
y
Ɵ
R
40 N
60 N
F = 300 N
W = 100 N
T = 100 N
60°
30°
F
x
F
y
T
x
T
y
Topic 1: Vectors In Two Dimensions
6.3
(3)
7
x 56
22
2
=+
,xkm61 781
D
==
() , °tantan
5
6
5
6
50 19
1
i
== =
-
,,xkmbearing781 140 19
=
c
(8)
8.1
.(). ,
°°
coscosFF N30 100 30 86 60
x
== =
right
.().
°°
si
ns
inFF N30 100 30 50
y
== =
upwards
vector diagram
(6)
8.2
.().°°coscosxx20 280 20
D
==
= 263,11m east
.().°°sinsinyx20 280 20
D
==
= 95,77 m north
vector diagram
(6)
8.3
.().°°coscosFF 40 400 40
x
==
= 306,42N right
.().°°sinsinFF40 400 40
y
==
= 257,11N upwards
vector diagram
(6)
8.4
.().°°coscosFF 40 500 40
x
==
= 383,02N right
.().°°sinsinFF40 500 40
y
==
= 321,39N
downwards
vector diagram
(6)
9.1
(). °cosF 300 60
x
=
= 150N right
().
°
sinF 300 60
y
=
=259,81N up
(6)
9.2
(). °cosT 100 30
x
=
=86,60 N left
(). °sinT 100 30
y
=
=50N up
(6)
7
Grade 11 PHYSICAL SCIENCES Term 1
2 km
8 km
5 km
P
Δx
P
Ɵ
30°
F
x
F
y
20°
x
y
40°
F
x
F
y
40°
F
x
F
y
Ɵ
R
40 N
60 N
F = 300 N
W = 100 N
T = 100 N
60°
30°
F
x
F
y
T
x
T
y
Topic 1: Vectors In Two Dimensions
WORKSHEETS
6.2
6
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: WORKSHEET MEMORANDUM
1. Choose forward as the positive (+) direction.
Then:
RN50 180 130 130
=+ +- =- =
^h
backwards (2)
2.
Choose east as the positive (+) direction.
Then:
()xm150 70 100 180
D
=+ +- +=
East (2)
3. Choose upwards as the positive (+) direction.
Then:
RN
500 300 600 200 200
=+ ++-=+=
^h
upwards (2)
4.1
Ɵ
8 km
12 km
Δx
(3)
4.2
,
() ,
,,
°
°
tantan
x
xk
m
xkmbearing
812
208 14 42
8
12
8
12
56 31
14 42 56 31
222
1
=+
==
== =
=
-
(5)
5.
(4)
6.1
RN20 80 60 60
x
=+ +- =- =
^h
left
RN50 90 40 40
y
=+ +- =- =
^h
downwards
(4)
6.2
40 N
60 N
Ɵ
R
40 N
60 N
R 60 40
222
=+
,RN5200 72 11
==
() , °tantan
60
40
60
40
33 69
1
i
== =
-
,,°RNat belowthe horizontal72 11 33 69
=
(7)
F
3
= 80 N
F
1
= 20
F
2
= 50 N
F
4
= 90 N
R 60 40
222
=+
,N
R 5 200 72 11
==
,tantan
60
40
60
40
33 69
1
` cii
== =
-
bl
, ,Natbelow thehorizontalR 72 11 33 69
°
=
(7)
6.3
6.3
(3)
7
x 56
22
2
=+
,xkm61 781
D
==
() , °tantan
5
6
5
6
50 19
1
i
== =
-
,,xkmbearing781 140 19
=
c
(8)
8.1
.(). ,
°°
coscosFF N30 100 30 86 60
x
== =
right
.().
°°
si
ns
inFF N30 100 30 50
y
== =
upwards
vector diagram
(6)
8.2
.().°°coscosxx20 280 20
D
==
= 263,11m east
.().°°sinsinyx20 280 20
D
==
= 95,77 m north
vector diagram
(6)
8.3
.().°°coscosFF 40 400 40
x
==
= 306,42N right
.().°°sinsinFF40 400 40
y
==
= 257,11N upwards
vector diagram
(6)
8.4
.().°°coscosFF 40 500 40
x
==
= 383,02N right
.().°°sinsinFF40 500 40
y
==
= 321,39N
downwards
vector diagram
(6)
9.1
(). °cosF 300 60
x
=
= 150N right
().
°
sinF 300 60
y
=
=259,81N up
(6)
9.2
(). °cosT 100 30
x
=
=86,60 N left
(). °sinT 100 30
y
=
=50N up
(6)
7
Grade 11 PHYSICAL SCIENCES Term 1
2 km
8 km
5 km
P
Δx
P
Ɵ
30°
F
x
F
y
20°
x
y
40°
F
x
F
y
40°
F
x
F
y
Ɵ
R
40 N
60 N
F = 300 N
W = 100 N
T = 100 N
60°
30°
F
x
F
y
T
x
T
y
Topic 1: Vectors In Two Dimensions
(3)
7.
x 56
222
=+
,kmx 61 781
T
==
,
tan
tan
5
6
5
6
50 19
1
c
{
i
i
=
==
-
ak
,,km on abearing ofx 781 140 19cT
=
(8)
8. 8.1
,coscos NrightFF 30 100 30 86 60
°°
x
{ {
== =
^h
sinsin NupwardsFF 30 100 30 50
°°
y
{ {
== =
^h
vector diagram (6)
8.2
,coscos meastxx20 280 20 263 11°°T{{
== =
^h
,sinsin mnorthyx20 280 20 95 77°°T{{
== =
^h
vector diagram (6)
8.3
,coscos NrightFF 40 400 40 306 42
°°
x
{ {
== =
^h
,sinsin NupwardsFF 40 400 40 257 11
°°
y
{ {
== =
^h
vector diagram (6)
8.4
..,coscos NrightFF 40 500 40 383 02
°°
x
{ {
== =
^h
..,sinsin NdownwardsFF 40 500 40 321 39
°°
y
{ {
== =
^h
vector diagram (6)
Gr11_Physical_Science_Term1_Resource_Book.indb 9 2018/12/24 8:21:53 AM
RESOURCE PACK
F = 300 N
W = 100
N
T = 100 N
60°
30°
F
x
F
y
T
x
T
y
R
y
= 209,81 N
y
= 209,81 N
y
R
x
= 63,4
x
= 63,4
x
N
Ɵ
R
R
9. 9.1
co
sN
rightF 300 60 150
°
x
{ {
==
^h
,si
nN
upF 300 60 259 81
°
y
{ {
==
^h
(4)
9.2
,co
sN
leftT 100 30 86 60
°
x
{ {
==
^h
si
nN
upT 100 30 50
°
y
{ {
==
^h
(4)
9.3
(,),
,(),
,,
,,
,
,
,
,
,
,,
tan
tan
Nright
Nup
N
Natabove thehorizontal
R
R
R
R
R
150 86 60 63 4
259 81 50 100 209 81
63 4 209 81
48 039 80 219 18
63 4
209 81
63 4
209 81
73 19
219 18 73 19
x
y
22 2
1
c
c
{
{
{
{
{
{
i
i
=+ +- =
=+ ++-=
=+
==
=
==
=
-
dn
vector diagram
(8)
Gr11_Physical_Science_Term1_Resource_Book.indb 10 2018/12/24 8:21:58 AM
9
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: CONSOLIDATION QUESTIONS MEMORANDUM TOTAL: 40 MARKS
A single vector which has the same eect as the original vectors acting together (2)
forces to
the right
forces to the left (2)
Right is positive (+):
RN10 30 15 25 10
=+ ++-+-=
^^hh
right (2)
x 812
222
D
=+
,
xk
m208 14 42D
==
Tan
8
12
i
=
() , °Tan
8
12
56 3
1
i
==
-
,,xkmbearing14 42 303 7
D
=
(5)
The resultant vertical force is:
RN40 10 30
y
=+ +- =
^h
upwards
The resultant horizontal force is:
RN60
x
=
left
R 60 30
222
=+
,RN4500 67 08
==
Tan
60
30
i
=
() , °Tan
60
30
26 6
1
i
==
-
,,°RNat67 08 26 6
=
above the horizontal (6)
()
.,°
TCos N500 45 353 55
x
==
right
()
.,°
TSin N500 45 353 55
y
==
up
(4)
()
.,°
FCos N300 20 281 91
x
==
left
()
.,°
FSin N300 20 102 61
y
==
up
(4)
,,,
RN
353 55 281 91 71 64
x
=+ +- =
^h
right
(2)
,, ,
RN
353 55 102 61 200 256 16
y
=+ ++-=
^h
up
(2)
,,R 71 64 256 16
22 2
=+
,,RN70750 23 265 99
==
,
,
Tan
71 64
256 16
i
=
(
,
,
),
°Tan
71 64
256 16
74 4
1
i
==
-
,,°RNat265 99 74 4
=
above the horizontal
(5)
()
.,°
FCos N1500 25 1359 46
x
==
right
()
.,°
FSin N1500 25 633 93
y
==
up
,, ,RN1359 46 1359 46 2718 92
x
=+ +=
right
,,R 633 93 633 93 0
y
=+ +- =
^h
,RN2718 92
=
at bearing 90°
(6)
[40]
15 N 10 N
30 N
25 N
Ɵ
8 km
12 km
Δx
60 N
30 N
Ɵ
R
30 N
60 N
T = 500 N
W = 200 N
F = 300 N
45°
20°
T
x
T
y
F
x
F
y
R
y
= 256,16 N
R
x
= 71,64 N
N
Ɵ
R
F = 1500 N
F = 1500 N
N
25°
25°
F
x
F
x
F
y
F
y
9
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: CONSOLIDATION QUESTIONS MEMORANDUM TOTAL: 40 MARKS
A single vector which has the same eect as the original vectors acting together (2)
forces to
the right
forces to the left (2)
Right is positive (+):
RN10 30 15 25 10
=+ ++-+-=
^^hh
right (2)
x 812
222
D
=+
,
xk
m208 14 42D
==
Tan
8
12
i
=
() , °Tan
8
12
56 3
1
i
==
-
,,xkmbearing14 42 303 7
D
=
(5)
The resultant vertical force is:
RN40 10 30
y
=+ +- =
^h
upwards
The resultant horizontal force is:
RN60
x
=
left
R 60 30
222
=+
,RN4500 67 08
==
Tan
60
30
i
=
() , °Tan
60
30
26 6
1
i
==
-
,,°RNat67 08 26 6
=
above the horizontal (6)
()
.,°
TCos N500 45 353 55
x
==
right
()
.,°
TSin N500 45 353 55
y
==
up
(4)
()
.,°
FCos N300 20 281 91
x
==
left
()
.,°
FSin N300 20 102 61
y
==
up
(4)
,,,
RN
353 55 281 91 71 64
x
=+ +- =
^h
right
(2)
,, ,
RN
353 55 102 61 200 256 16
y
=+ ++-=
^h
up
(2)
,,R 71 64 256 16
22 2
=+
,,RN70750 23 265 99
==
,
,
Tan
71 64
256 16
i
=
(
,
,
),
°Tan
71 64
256 16
74 4
1
i
==
-
,,°RNat265 99 74 4
=
above the horizontal
(5)
()
.,°
FCos N1500 25 1359 46
x
==
right
()
.,°
FSin N1500 25 633 93
y
==
up
,, ,RN1359 46 1359 46 2718 92
x
=+ +=
right
,,R 633 93 633 93 0
y
=+ +- =
^h
,RN2718 92
=
at bearing 90°
(6)
[40]
15 N 10 N
30 N
25 N
Ɵ
8 km
12 km
Δx
60 N
30 N
Ɵ
R
30 N
60 N
T = 500 N
W = 200 N
F = 300 N
45°
20°
T
x
T
y
F
x
F
y
R
y
= 256,16 N
R
x
= 71,64 N
N
Ɵ
R
F = 1500 N
F = 1500 N
N
25°
25°
F
x
F
x
F
y
F
y
9
Grade 11 PHYSICAL SCIENCES Term 1
Topic 1: Vectors In Two Dimensions
GRADE 11: CONSOLIDATION QUESTIONS MEMORANDUM TOTAL: 40 MARKS
A single vector which has the same eect as the original vectors acting together (2)
forces to
the right
forces to the left (2)
Right is positive (+):
RN10 30 15 25 10
=+ ++-+-=
^^hh
right (2)
x 812
222
D
=+
,
xk
m208 14 42D
==
Tan
8
12
i
=
() , °Tan
8
12
56 3
1
i
==
-
,,xkmbearing14 42 303 7
D
=
(5)
The resultant vertical force is:
RN40 10 30
y
=+ +- =
^h
upwards
The resultant horizontal force is:
RN60
x
=
left
R 60 30
222
=+
,RN4500 67 08
==
Tan
60
30
i
=
() , °Tan
60
30
26 6
1
i
==
-
,,°RNat67 08 26 6
=
above the horizontal (6)
()
.,°
TCos N500 45 353 55
x
==
right
()
.,°
TSin N500 45 353 55
y
==
up
(4)
()
.,°
FCos N300 20 281 91
x
==
left
()
.,°
FSin N300 20 102 61
y
==
up
(4)
,,,
RN
353 55 281 91 71 64
x
=+ +- =
^h
right
(2)
,, ,
RN
353 55 102 61 200 256 16
y
=+ ++-=
^h
up
(2)
,,R 71 64 256 16
22 2
=+
,,RN70750 23 265 99
==
,
,
Tan
71 64
256 16
i
=
(
,
,
),
°Tan
71 64
256 16
74 4
1
i
==
-
,,°RNat265 99 74 4
=
above the horizontal
(5)
()
.,°
FCos N1500 25 1359 46
x
==
right
()
.,°
FSin N1500 25 633 93
y
==
up
,, ,RN1359 46 1359 46 2718 92
x
=+ +=
right
,,R 633 93 633 93 0
y
=+ +- =
^h
,RN2718 92
=
at bearing 90°
(6)
[40]
15 N 10 N
30 N
25 N
Ɵ
8 km
12 km
Δx
60 N
30 N
Ɵ
R
30 N
60 N
T = 500 N
W = 200 N
F = 300 N
45°
20°
T
x
T
y
F
x
F
y
R
y
= 256,16 N
R
x
= 71,64 N
N
Ɵ
R
F = 1500 N
F = 1500 N
N
25°
25°
F
x
F
x
F
y
F
y
WORKSHEETS
CONSOLIDATION EXERCISE
TOTAL: 40 MARKS
1. A single vector which has the same eect as two or more vectors acting together OR
e vector sum of two or more vectors. (2)
2. 2.1
forces to the right
forces to the le (2)
2.2 Right is positive (+):
NR 10 30 15 25 0
{ {
=+++-+-=
^^hh
(2)
3.
81
2
2
22
x
=+
T
,k
mx 208 14 42T
==
tan
8
12
i
=
,tan
8
12
56 3°
1
i
==
-
ak
,,km on abearing ofx 14 42 303 7T
=
(5)
4. e resultant vertical force is:
NupwardsR 40 10 30
y
=+ +- =
^h
e resultant horizontal force is:
NleftR 60
x
=
R 60 30
222
=+
,NR 4 500 67 08
==
tan
60
30
i
=
,tan
60
30
26 6°
1
i
==
-
ak
, ,Natabove thehorizontalR 67 08 26 6c
=
(6)
5. 5.1
,cosNrightT 500 45 353 55°
x
{ {
==
^h
,sinNupT 500 45 353 55°
y
{ {
==
^h
(4)
5.2
,cosNleftF 300 20 281 91°
x
{ {
==
^h
,sinNupF 300 20 102 61°
y
{ {
==
^h
(4)
5.3
,,,NrightR 353 55 281 91 71 64
x
{ {
=+ +- =
^h
(2)
5.4
,,() ,NupR 353 55 102 61 200 256 16
y
{ {
=+ ++-=
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 11 2018/12/24 8:22:07 AM
RESOURCE PACK
5.5
,,R 71 64 256 16
22 2
=+
,,NR 70 750 33 265 99
==
,
,
tan
71 64
256 16
i
=
,
,
,tan
71 64
256 16
74 4
1
ci
==
-
cm
,,Natabove thehorizontalR 265 99 74 4
c
{ {
=
(5)
6.
, cosNrightF 1 500 25 1 359 46°
x
==
^h
, sinNupwardsF 1 500 25 633 93°
y
==
^h
,, ,R 1 359 46 1 359 46 2 718 92
x
=+ +=
,,R 633 93 633 93 0
y
=+ +- =
^h
, Natbearing ofR 2 718 92 090
c
=
(6)
[40]
Gr11_Physical_Science_Term1_Resource_Book.indb 12 2018/12/24 8:22:11 AM
WORKSHEETS
Topic 2 – Newton’s Laws and
Application of Newton’s Laws
WORKSHEET
1. A 3 kg box is resting on a horizontal surface. e coecient of static friction between
the surface and the box is 0,34.
1.1 Calculate the weight of the box. (2)
1.2 Calculate the maximum force of static friction between the box and the surface. (3)
2. Rugby players are trying to push a 500 kg scrum machine along horizontal ground. e
coecient of static friction between the scrum machine and the ground is 0,65.
2.1 Calculate the normal force acting on the scrum machine. (2)
2.2 If the players exert a horizontal force of 3 000 N on the scrum machine, will the
scrum machine move? Give a reason for your answer. (3)
3. e brakes on a 20 kg cart are locked so that the rubber wheels cannot turn. A child
pushes horizontally to the right on the cart until it just begins to slide. e maximum
horizontal force he applies is 150 N. Aer that the child is able to keep the cart sliding
with a horizontal force of 80 N.
3.1 Dene a normal force. (2)
3.2 Explain the dierence between static and kinetic friction. (2)
3.3 Calculate the coecients of static and kinetic friction between the rubber wheels
and the ground. (5)
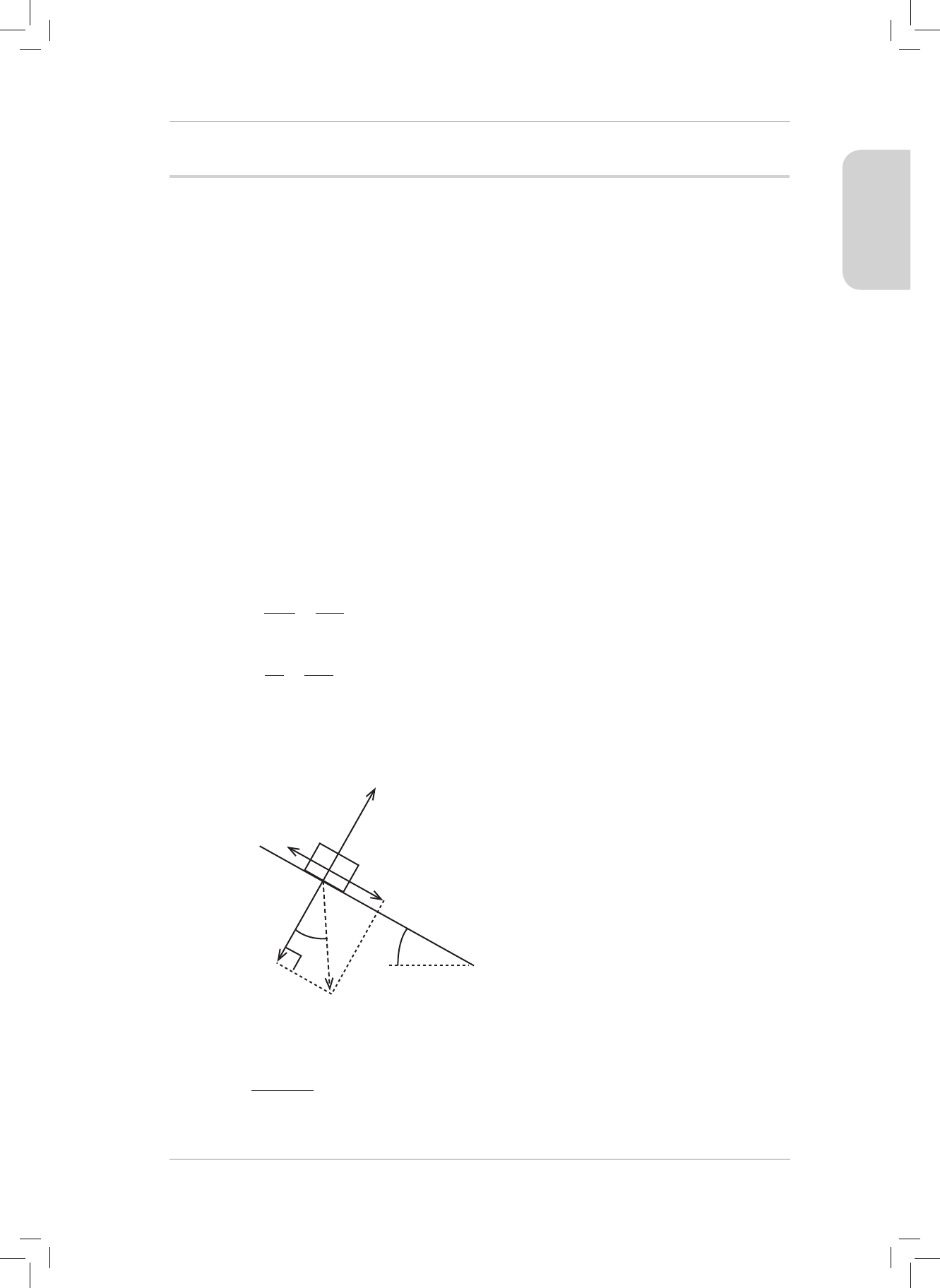
4. A 5 kg box is resting on a plane inclined at 30° to the horizontal. e coecient of static
friction between the box and the plane is 0,63.
4.1 Dene a frictional force. (2)
4.2 Draw a neat, fully labelled force diagram showing all the forces acting on the box.
Now draw and label the horizontal (w
x
) and vertical (w
y
) components of the weight
(w) of the box. Label the 30° angle. (6)
4.3 Calculate the maximum static frictional force between the box and the inclined
plane. (4)
4.4 Use a calculation to explain why the box does not slide down the slope. (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 13 2018/12/24 8:22:11 AM
RESOURCE PACK
5. A 50 N force is applied to a trolley at an angle of 35° to the horizontal.
50 N
35˚
Calculate the normal force acting on the trolley if the mass of the trolley is 6 kg. (4)
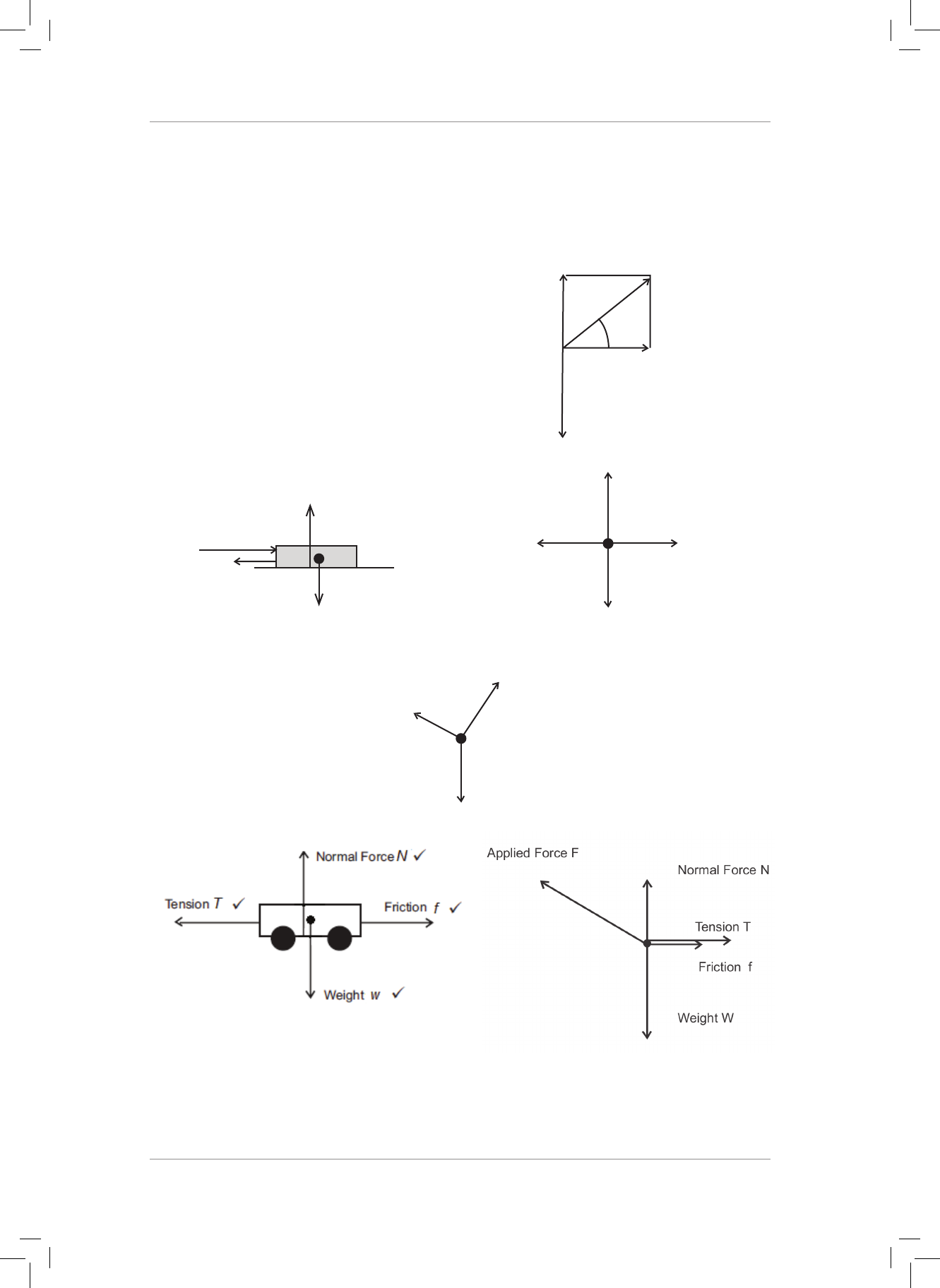
6. 6.1 Draw a fully labelled force diagram for the scrum machine in Question 2 (above)
while it is sliding along the ground. (5)
6.2 Draw a fully labelled free-body diagram for the cart in Question 3 (above) before it
starts to move. (5)
6.3 Draw a fully labelled free-body diagram for the box in Question 4 (above). (3)
7. Two trolleys of mass 2 kg and 1 kg are joined together by a light string. A force 25 N is
applied to the 2 kg trolley at 30° to the horizontal. e system moves to the le along a
rough horizontal surface as shown in the diagram below.
25 N
1 kg
Rough surface
2 kg
30°
7.1 Draw a fully labelled force diagram for the 1 kg trolley. (4)
7.2 Draw a fully labelled free-body diagram for the 2 kg trolley. (5)
8. State Newton’s rst law of motion. (2)
9. Explain what is meant by the term “inertia”. (2)
10. List three dierent types of motion and state whether or not a net force is acting during
each type of motion. (6)
11. What property of an object determines how much inertia it has? (1)
12. A teacher is walking along the corridor carrying a full cup of coee, when a student
runs in front of her. She stops suddenly and spills her coee. Use Newton’s rst law to
explain why the coee spills forwards when the teacher stops suddenly. (3)
13. Many car passengers have suered neck injuries when struck by cars from behind. Use
Newton’s rst law to explain how headrests help to guard against this type of injury. (4)
Gr11_Physical_Science_Term1_Resource_Book.indb 14 2018/12/24 8:22:14 AM
WORKSHEETS
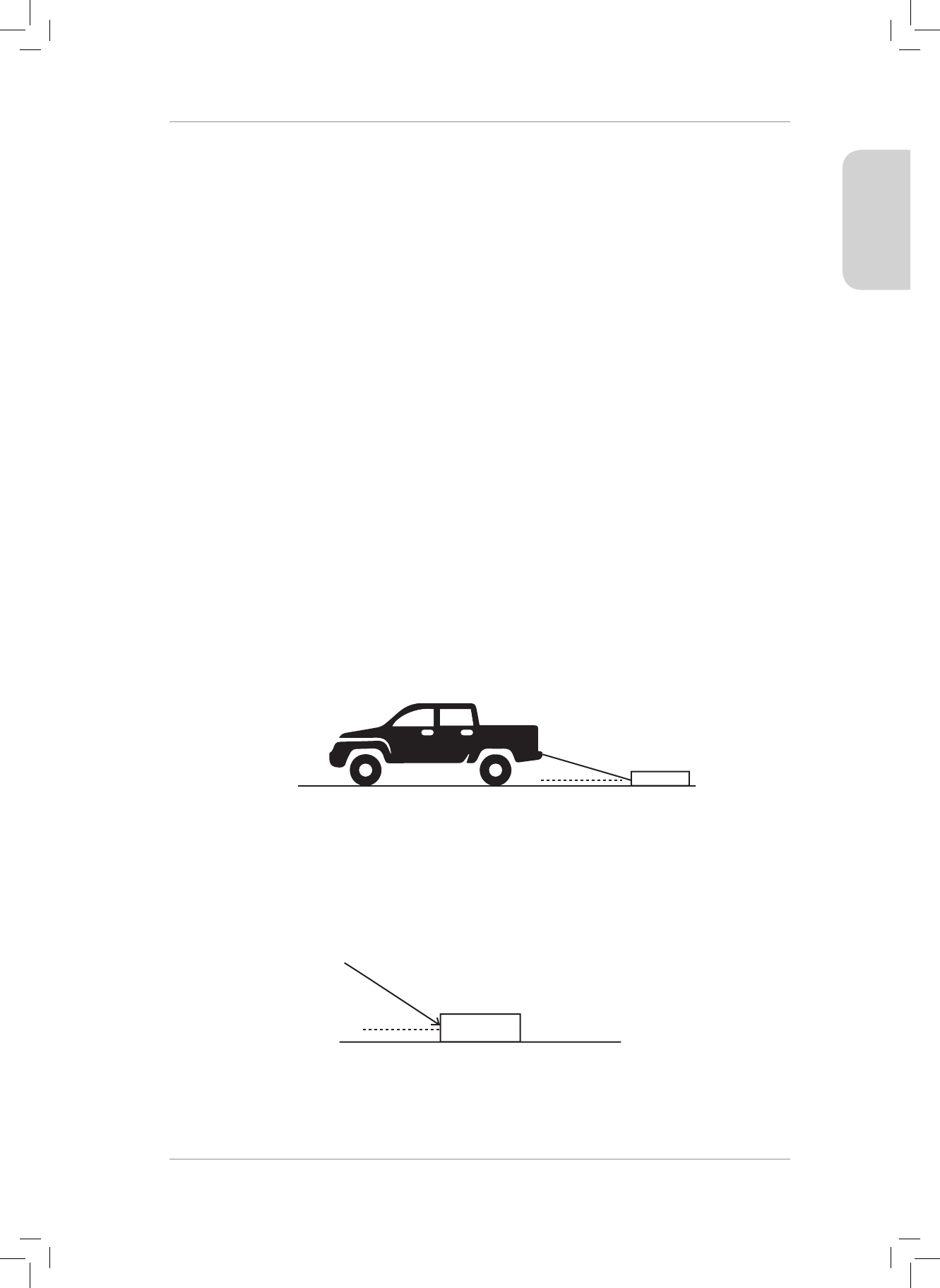
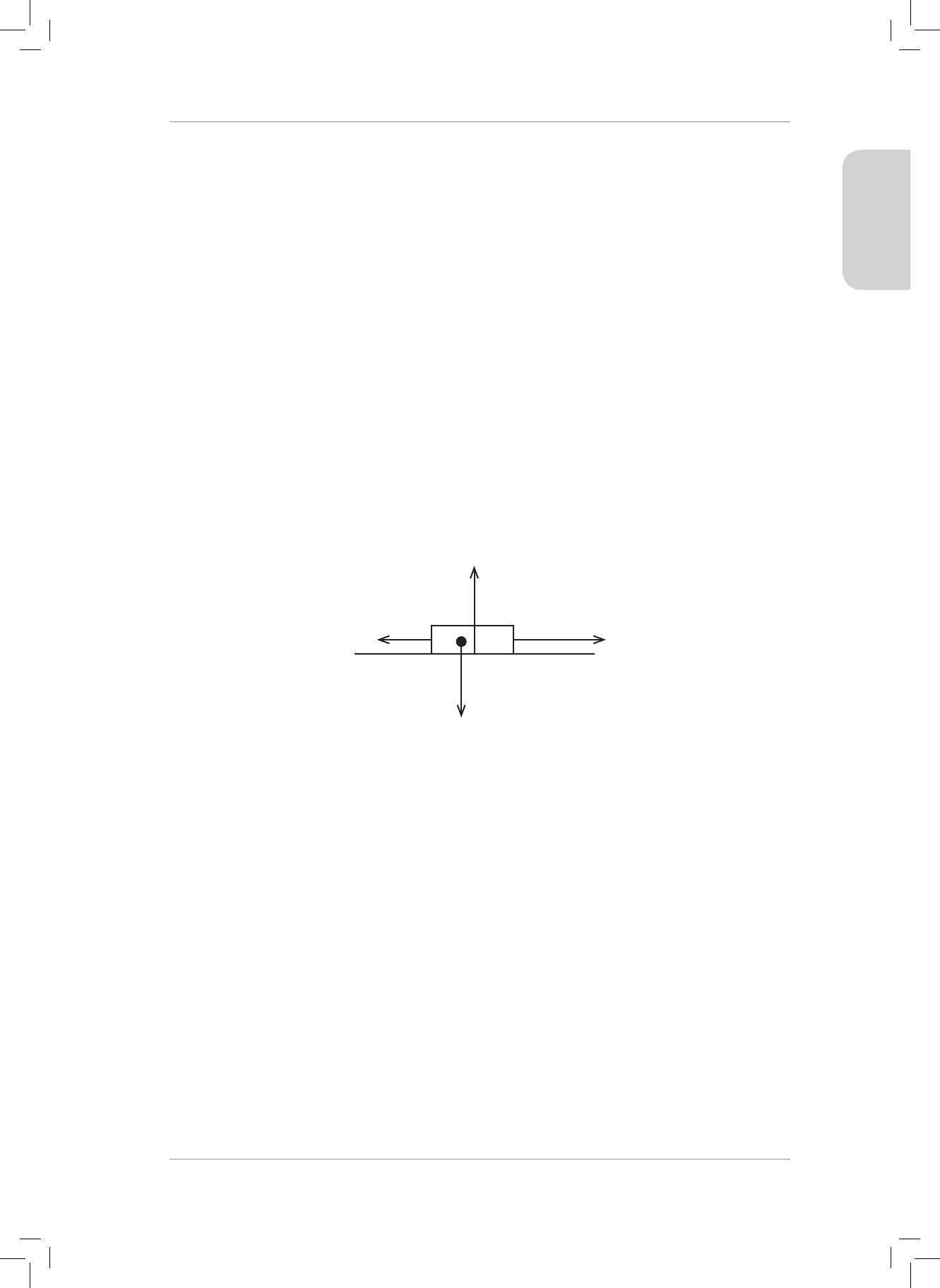
14. A 8 kg box is placed on a table. A horizontal force of magnitude 68 N is applied to the
right on the box. A frictional force of magnitude 27 N is present between the surface
and the box.
14.1 Draw a force diagram indicating all of the forces acting on the box. (4)
14.2 Calculate the acceleration of the box. (4)
15. Two crates, 4 kg and 6 kg respectively, are connected with a thin inextensible rope. A
force of 89 N is applied to the right. e frictional forces on the 4 kg and 6 kg are 19,6 N
and 29,4 N respectively.
15.1 Calculate the acceleration of the crates. (6)
15.2 Calculate the magnitude of the tension T in the rope that connects the boxes. (2)
16. A man is pulling a 30 kg box to the le along a rough horizontal plane with an
inextensible rope that makes an angle of 45° above the horizontal. He applies a force of
250 N and the coecient of kinetic friction between the box and the surface is 0,6.
16.1 Draw a fully labelled force diagram for the box. (4)
16.2 Calculate the kinetic frictional force acting on the box. (5)
16.3 Calculate the acceleration of the box. (5)
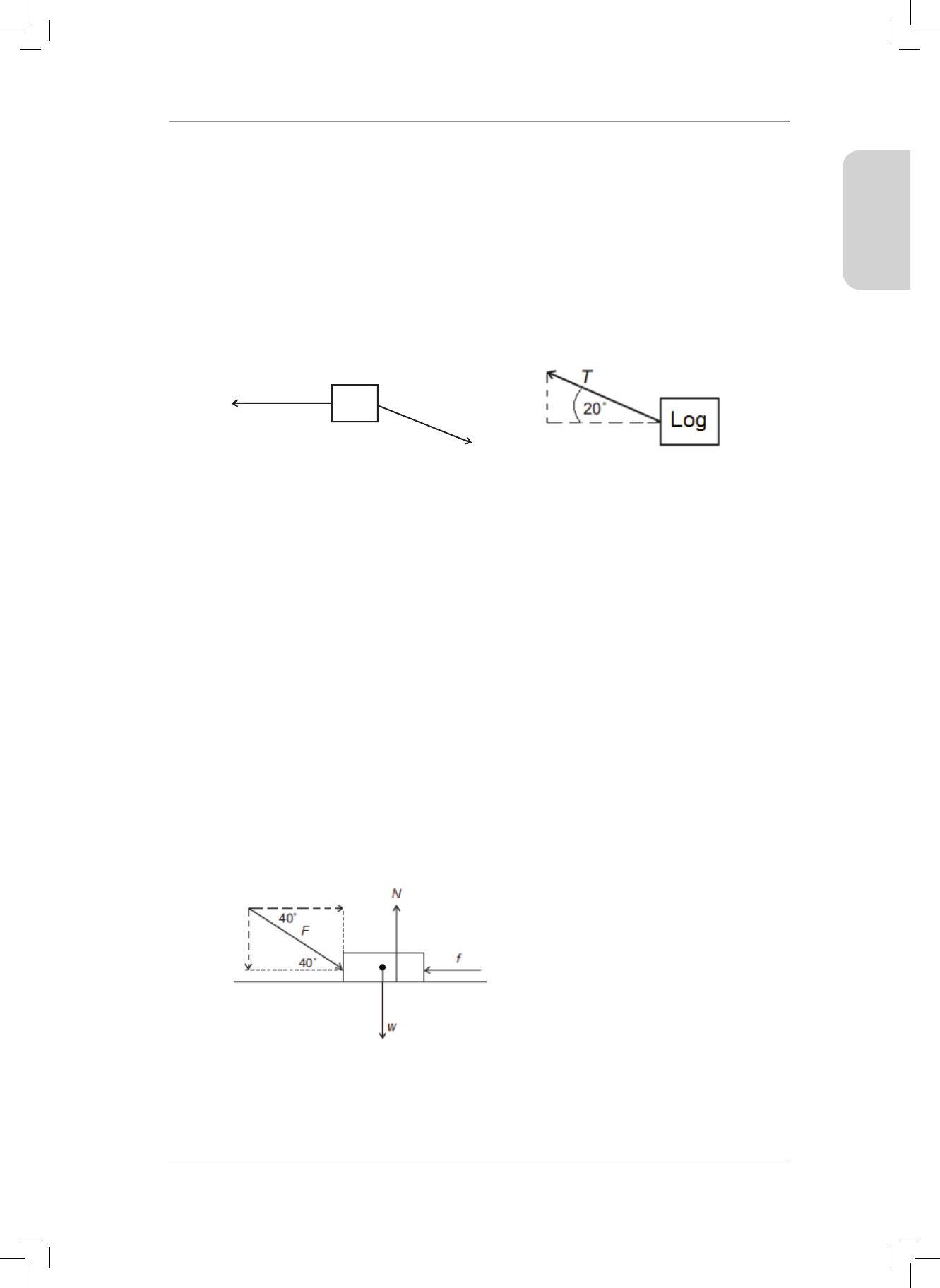
17. A 2 000 kg truck pulls a 200 kg log with a constant acceleration. e engine of the truck
produces a forward force of 10000 N. e tow rope makes an angle of 20° with the
horizontal. Ignore the eect of friction, and the mass of the tow rope. e tow rope is
inextensible.
T
20˚
Calculate the:
17.1 acceleration of the truck; and (7)
17.2 magnitude of the tension T in the tow rope between the truck and the log. (2)
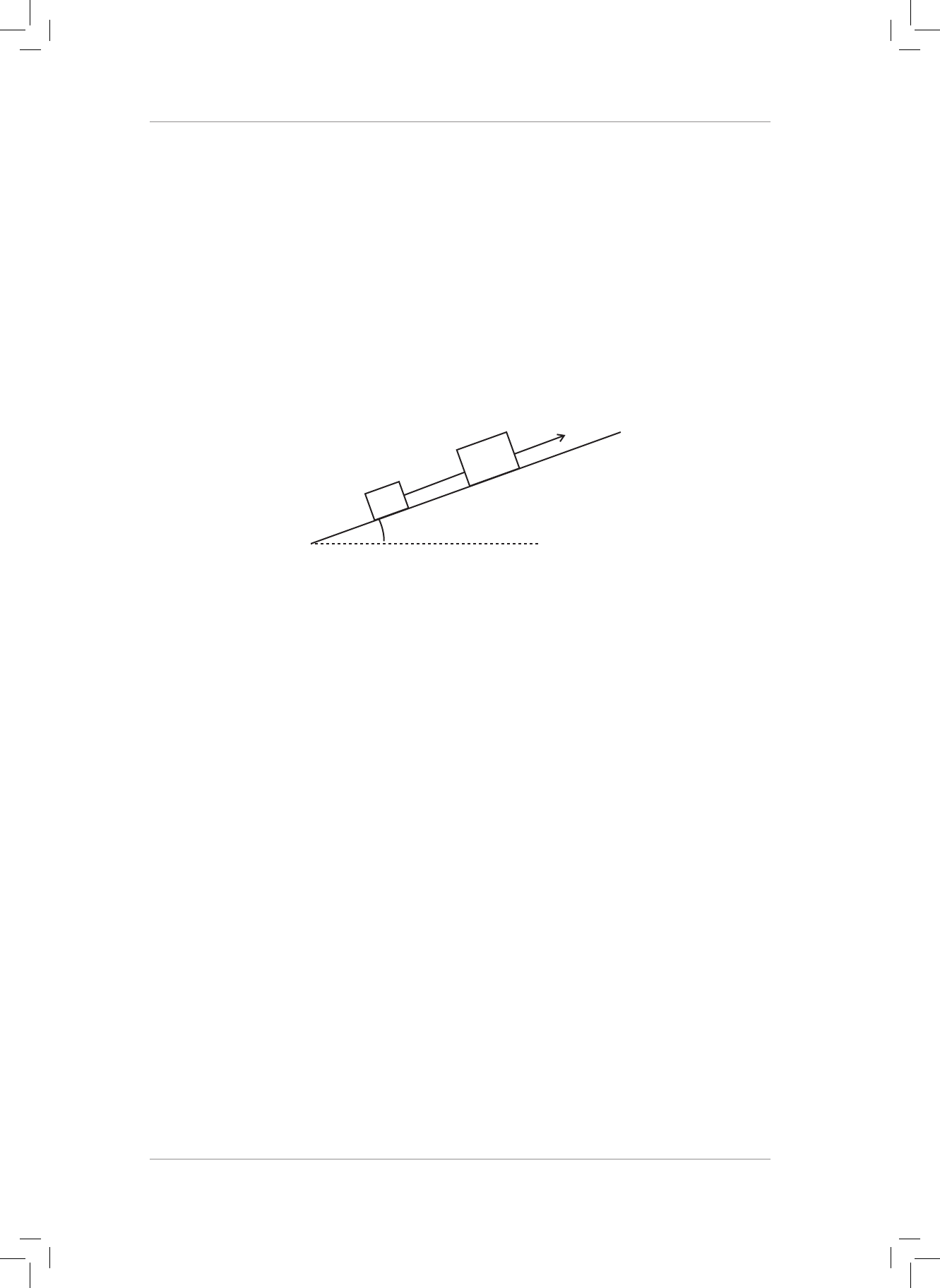
18. A force of 300 N, acts downwards on a 30 kg block at 40° to the horizontal as shown in
the diagram below. e block accelerates to the right along a rough horizontal surface.
F = 300 N
40˚
18.1 Calculate the magnitude and direction of the component of the 300 N force that
accelerates the block horizontally. (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 15 2018/12/24 8:22:15 AM
RESOURCE PACK
18.2 If the acceleration of the block is 2 m·s
−2
, calculate the magnitude of the frictional
force acting on the block. (4)
18.3 Calculate the vertical force exerted by the block on the plane. (4)
19. A rocket (of mass 2 000 kg) is launched vertically upwards with an acceleration of
20m·s
−2
. Calculate the magnitude and direction of the thrust of the rocket’s engines. (4)
20. Two crates, 3 kg and 5 kg respectively, are connected with a thin rope. ey are pulled
up a rough plane which is inclined at 30° to the horizontal. A force F is applied parallel
to the plane as shown in the diagram accelerating the system of crates at 2 m·s
−2
up
the slope. e frictional forces acting on the 3 kg and 5 kg crates are 10 N and 17 N
respectively.
3 kg
30°
T
F
5 kg
20.1 Draw a fully labelled free-body diagram for each crate. (9)
20.2 Calculate the component of the weight of each crate parallel to the slope. (4)
20.3 Calculate the magnitude of the applied force F and the tension T in the rope. (4)
21. State Newton’s third law of motion. (2)
22. Identify two action-reaction pairs of forces present in each of the following situations:
22.1 A boy pushes a car along the road. (4)
22.2 A light bulb hangs from the ceiling by means of a cord. (4)
22.3 A ball is thrown up into the air. (4)
22.4 A donkey pulls a cart. (4)
23. State Newton’s law of Universal Gravitation. (3)
24. e two moons of Mars are Phobos and Deimos.
Mass of Phobos = 1,1 × 10
16
kg
Radius of Phobos = 11,1 km.
e distance between the centres of Mars and Phobos is 9377 km.
Mass of Mars = 6,4 × 10
23
kg.
24.1 Calculate the gravitational force of Mars on Phobos. (4)
24.2 Calculate the magnitude of the gravitational acceleration on the surface of Phobos.
(3)
24.3 By what factor would the acceleration due to gravity dier on the surface of
Deimos, which has half the radius of Phobos and one tenth of its mass? (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 16 2018/12/24 8:22:16 AM

WORKSHEETS
25. e James Webb Space Telescope will have a mass of 6 500 kg and will orbit the Earth
(mass 6×10
24
kg) approximately 1 500 000 km away from its centre. Calculate the
magnitude of the Earth’s gravitational force on the telescope. (3)
26. Mars One is an organisation based in the Netherlands that has put forward plans to land
the rst humans on Mars. ey aim to establish a permanent human colony there by
2027, with no plan of returning to Earth. e average distance between the centres of
Earth and Mars is 2,25×10
8
km. e planet has a mass of 6,42×10
23
kg, and a radius of
3380 km.
26.1 Calculate the magnitude of the acceleration due to gravity on the surface of Mars. (3)
26.2 Calculate the magnitude of the weight of a 500 kg spaceship on Mars. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 17 2018/12/24 8:22:16 AM
RESOURCE PACK
CONSOLIDATION EXERCISE
TOTAL: 63 MARKS
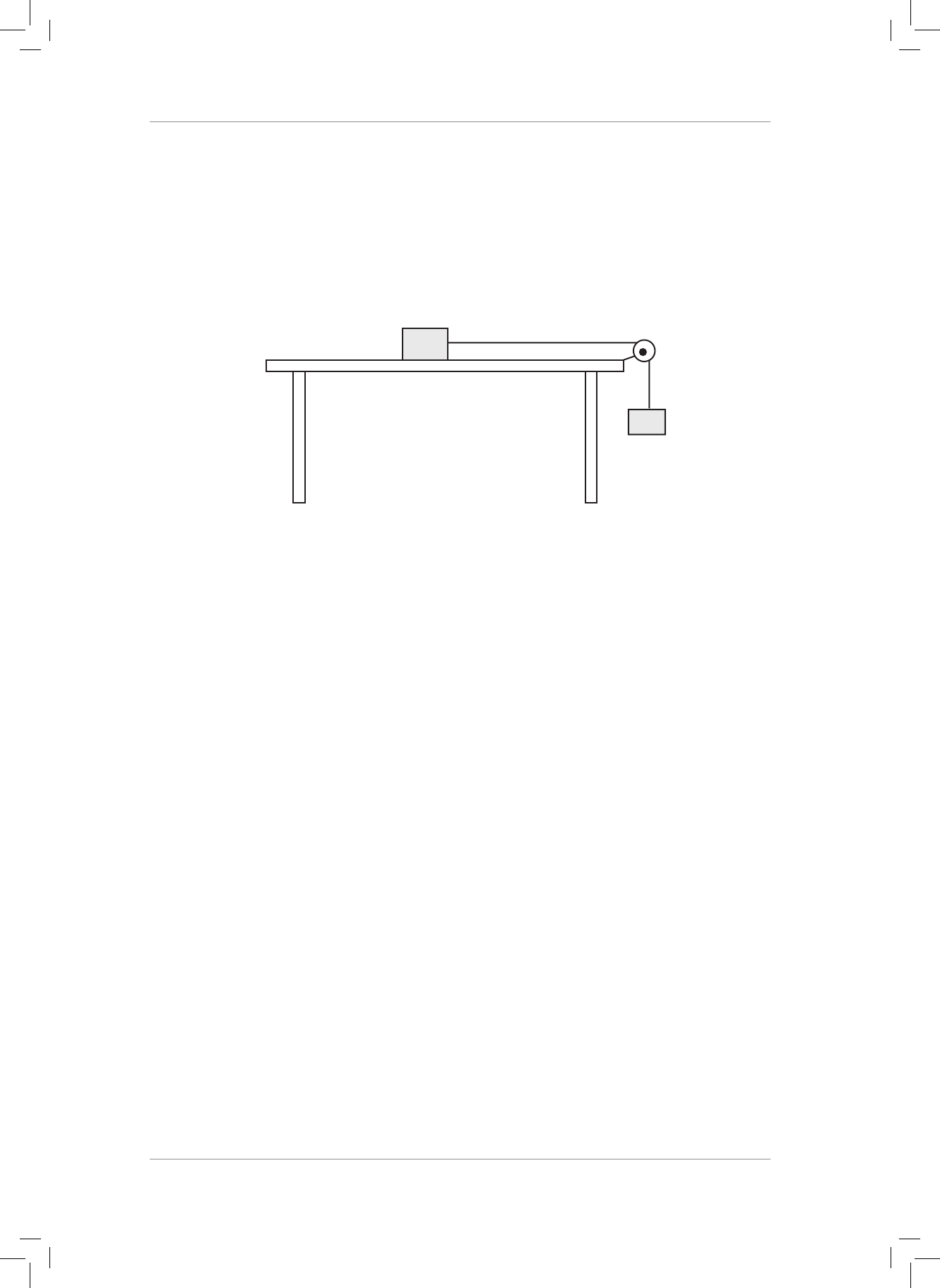
1. Two blocks (A and B) are connected by light string and set up as shown in the diagram
below.
A
T
B
3 kg
2 kg
e string passes over a frictionless pulley. e coecients of static and kinetic friction
are 0,40 and 0,28 respectively. When the system of masses is released, block A slides
across the table.
1.1 Dene a frictional force. (2)
1.2 Calculate the magnitude of the maximum static frictional force acting on block A. (2)
1.3 Calculate the force of kinetic friction acting on the 3 kg block. (3)
1.4 Draw a fully labelled free-body diagram for each block. (6)
1.5 State Newton’s second law. (3)
1.6 Calculate the acceleration of the system of blocks and the magnitude of the tension
T in the string. (6)
Gr11_Physical_Science_Term1_Resource_Book.indb 18 2018/12/24 8:22:17 AM
WORKSHEETS
2. A 1000 kg roller coaster free-wheels down a track that is inclined at 60° to the
horizontal.
60˚
2.1 Draw a fully labelled free-body diagram of all the forces acting on the roller coaster. (3)
2.2 Calculate the parallel and perpendicular components of the weight of the roller
coaster with respect to the inclined track. (4)
2.3 e coecient of friction between the wheels and track is 0,3. Calculate the force of
friction acting on the roller coaster. (3)
2.4 How would the force of friction be aected if the mass of the roller coaster was
increased? Explain your answer. (3)
2.5 Calculate the net force acting on the roller coaster. (3)
2.6 How would the magnitude of the net force change if the angle of the inclined track
was decreased? Assume that friction remains constant. Explain your answer. (2)
2.7 Calculate the acceleration of the roller coaster. (4)
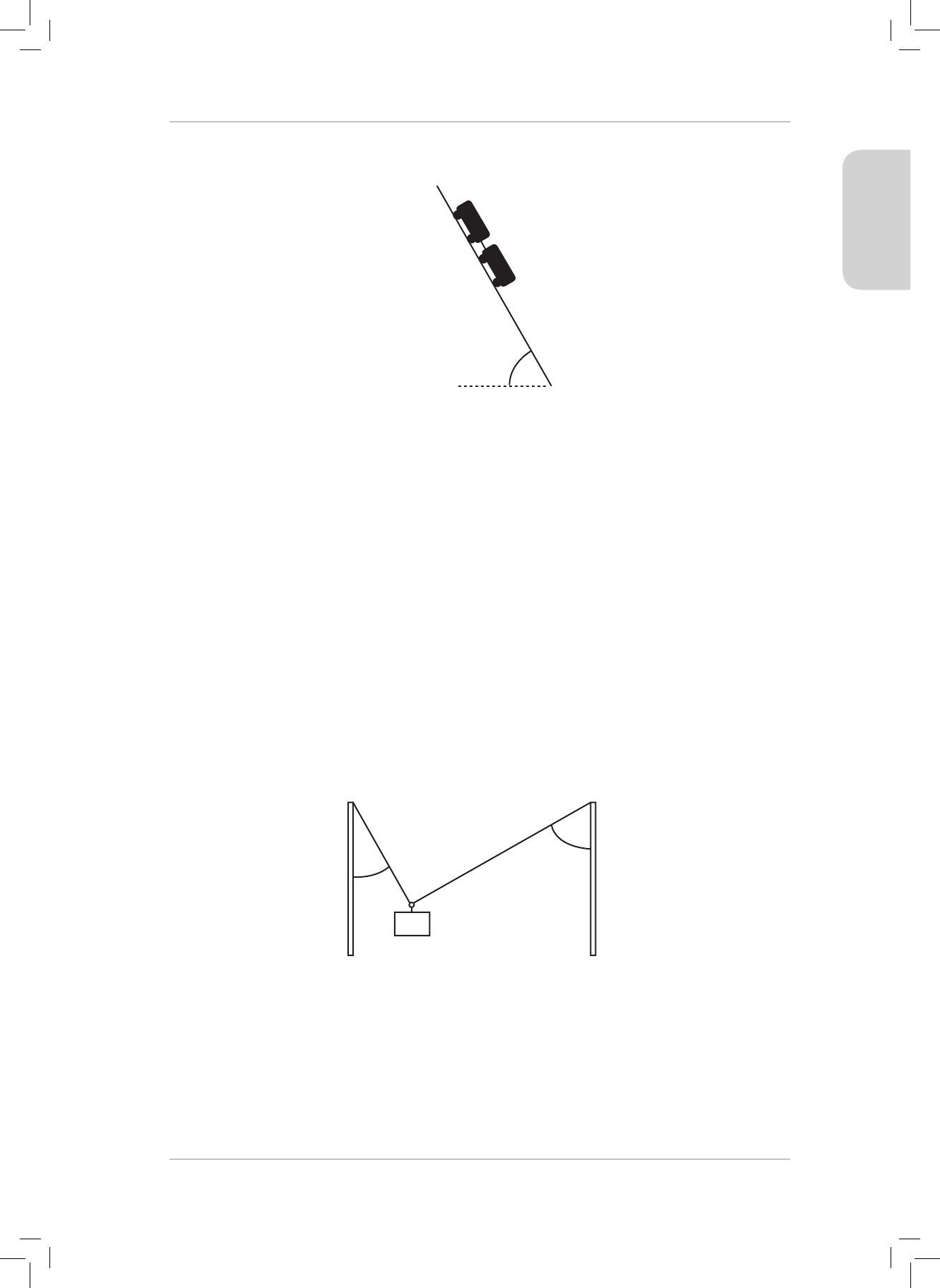
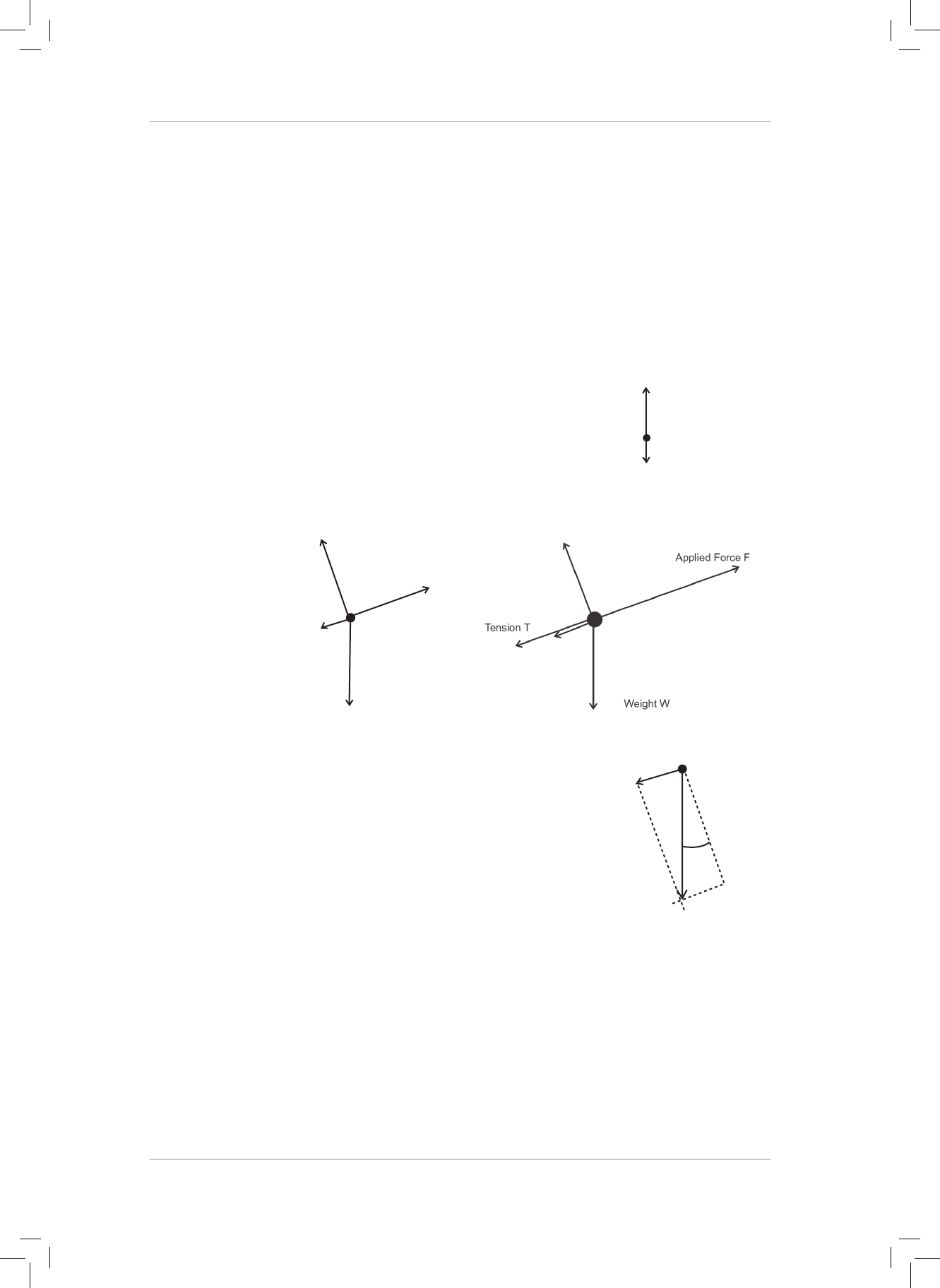
3. Two light ropes are strung between two vertical struts. A mass m of weight w hangs
from the ropes attached to a ring at point Y.
30˚
60˚
Q
P
Y
3.1 Draw a fully labelled free-body diagram of all the forces acting at point Y. (3)
3.2 Are the forces acting at point Y in equilibrium? Explain your answer. (2)
3.3 e tension in rope P is 600 N. Calculate the tension in rope Q. (4)
3.4 Calculate the mass m hanging from the two ropes. (4)
Gr11_Physical_Science_Term1_Resource_Book.indb 19 2018/12/24 8:22:19 AM
RESOURCE PACK
20
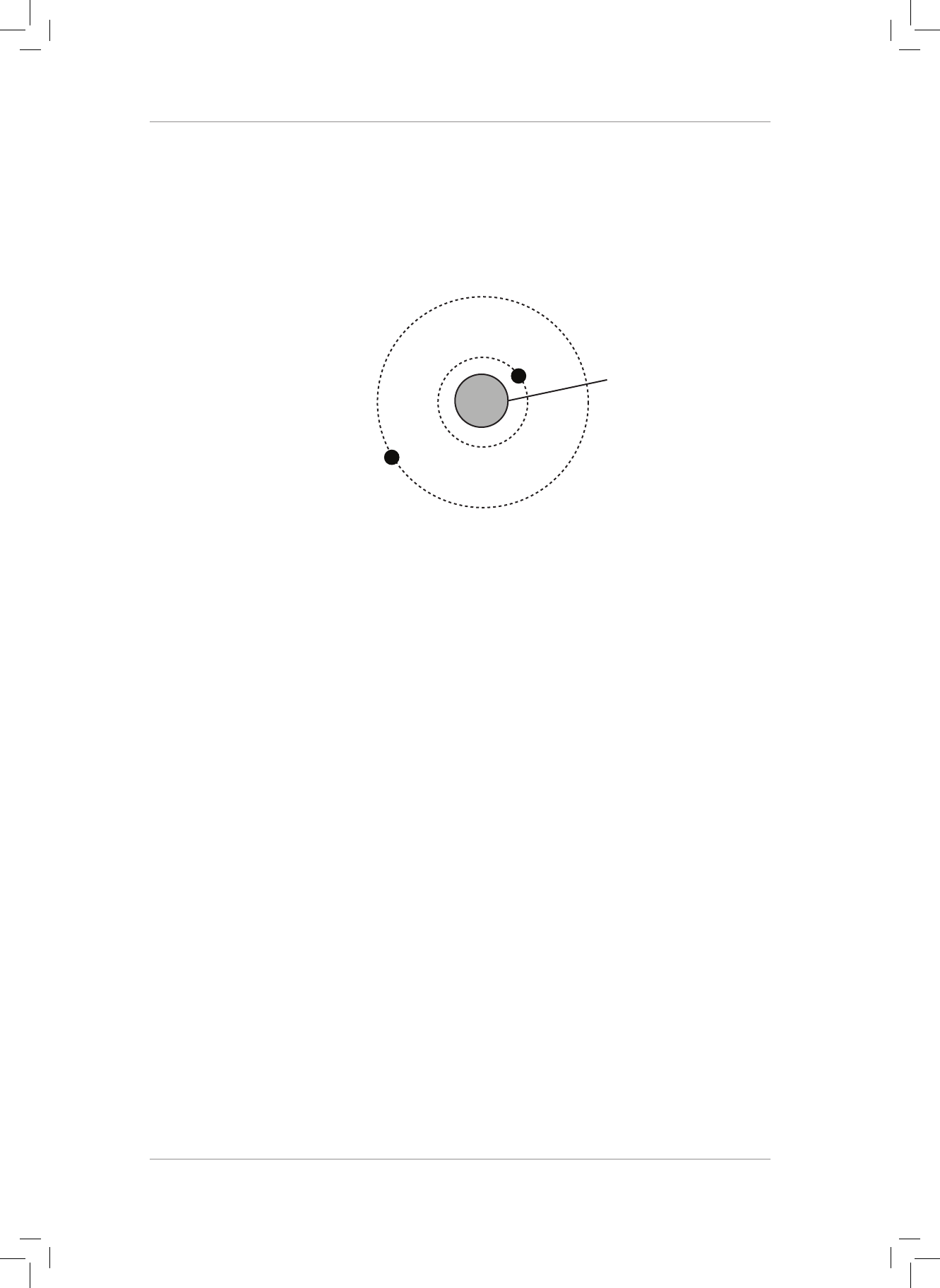
4. e diagram below shows the circular orbits of two of Jupiter’s moons: Adrastea A and
Megaclite, M.
Use the following data in your calculations.
Orbital radius of A = 1,3 x 10
8
m
e acceleration due to gravity at A = 7,5 m·s
−2
M
A
Jupiter
not to scale
4.1 Calculate the mass of Jupiter. (3)
4.2 If the value of g at Adrastea’s position is 34 090 times bigger than that at Megaclite’s
position, calculate the orbital radius of Megaclite without using the given value of g
at A or the mass of Jupiter that you calculated in question 4.1. (3)
[63]
Gr11_Physical_Science_Term1_Resource_Book.indb 20 2018/12/24 8:22:20 AM
WORKSHEETS
MARKING GUIDELINES
1.1
,, Ndownwmg 3982940
{
== =
^^hh
(2)
1.2
,,
,N
fN034294 10 00
max
ss
{
n
== =
^^hh
(3)
2.1
,N
wmg 500 98 4 900
{
== =
^^hh
erefore
Nupand perpendiculartothe groundN 4 900
{
=
(2)
2.2
,N
fN065 4900 3 185
max
ss
{
n
== =
^^hh
e machine will not move because the applied force of 3 000 N
is less than
f
max
s
{
(3)
3.1 e force exerted by a surface on an object in contact with it. (2)
3.2 Static friction is the frictional force that occurs between a stationary object and the
surface on which it is resting , whereas kinetic friction occurs when an object
slides across a surface. (2)
3.3
,Nwmg 20 98 196
== =
^^hh
erefore
NN 196 {
=
fN
max
ss
n
=
,
N
f
196
150
077
max
s
s
{
{
n
== =
fN
kk
n
=
,
N
f
196
80
041
k
k
{
{
n
== =
Coecients of static and kinetic friction do NOT have a unit. (5)
4.1 e force that opposes the motion of an object and acts parallel to the surface
the object is in contact with. (2)
4.2
e magnitude of the normal
force = the magnitude of w
y
.
e magnitude of the normal
force = the magnitude of w
x
.
30˚
30˚
Friction
F
s
w
x
w
y
Normal Force N
Direction of weight W
NB: Weight should not be shown as a vector because its components have been
included in the diagram. However, we need to show the angle 30° so we can show
the direction of weight.
(6)
Gr11_Physical_Science_Term1_Resource_Book.indb 21 2018/12/24 8:22:25 AM
RESOURCE PACK
22
PAGE 22 QUESTION 6 6.1 AND 6.2 DIAGRAMS
PAGE 22 QUESTION 7 DIAGRAM
PAGE 23 QUESTION 14.1 DIAGRAM
4.3
,,
coscos co
sN
Nw wmg30 30 598304244
°° °
y
{ {
== == =
^^hh
,,
,N
fN06342442673
max
ss
{ {
n
== =
^^hh
(4)
4.4
,,
sinsin si
nN
ww mg30 30 598302450
°° °
x
{ {
==
==
^^hh
fw
>
max
sx
{
(3)
5.
,sinsin NupFF 35 50 35 28 68°°
y
{
== =
^h
,,Ndownwmg 6985880 {
== =
^^hh
,, ,NdownF 58 80 28 68 30 12
down
{
=-=
, NupN 30 12
{
=
W
F = 50 N
(4)
6. 6.1 6.2
Friction f
K
Normal Force N
Weight w
Applied Force F
Static Friction f
s
Normal Force N
Weight w
Applied Force F
Applied force > Friction (5) Applied force = Friction (5)
6.3
Static Friction F
s
Normal Force N
Weight w
(3)
7. 7.1 7.2
(5)
NB Normal force = weight
Tension can be equal to friction or tension can be greater than friction.
Deduct a mark for each of these errors. (4)
Gr11_Physical_Science_Term1_Resource_Book.indb 22 2018/12/24 8:22:37 AM
23
WORKSHEETS
8. An object continues in a state of rest or constant velocity unless it is acted upon by a net
force. (2)
9. A property of a body which resists any change in its state of motion. (2)
10. A body remains at rest. e net force is zero.
A body travels at constant velocity. e net force is zero.
A body accelerates. e net force is NOT zero. (6)
11. Mass (1)
12. e hand exerts a net backward force on the cup. is net force does not act on the
coee. According to Newton’s rst law, the coee will continue moving forward at
constant velocity and spill over the front of the cup. (3)
13. e back of the seat exerts a net forward force on the passenger’s back. is net
force does not act on the passenger’s head. According to Newton’s rst law, the head
will remain at rest (and will be le behind). e neck muscles would experience
tremendous strain as they pull the head forward. A head rest will exert a net force
forward on the head, so the entire body would accelerate forward. (4)
14. 14.1
Friction
f
Applied Force F = 68 N
Normal Force N
Weight w
(4)
14.2 Choose right as positive:
Fma
net
=
Ff ma
{
-=
a68 27 8 {
-=
^h
a41 8
=
^h
,a 513
=
m·s
−2
to the right
(4)
Gr11_Physical_Science_Term1_Resource_Book.indb 23 2018/12/24 8:22:39 AM
RESOURCE PACK
24
15. 15.1
Tf = 19,6 N
4 kg
T
F = 89 N
f = 29,4 N
6 kg
4 kg: 6 kg:
Choose right as positive:
,
,
Fma
Tf a
Ta
Ta
4
19 64
19 64
net
{
{
=
-=
-=
=+
^h
,
,
Fma
FT
fa
Ta
Ta
6
89 29 46
59
66
net
{
{
=
--=
-- =
=-
^h
(i) (ii)
Set equation (i) equal to equation (ii)
,,aa19 64 59 66
+= -
a10 40
=
a 4
=
m·s
−2
to the right (6)
15.2
,,NT 19 644356{ {
=+ =
^h
(2)
16. 16.1
Friction f
Tension T = 250 N
Normal Force N
Weight w
45˚
(4)
16.2
, sinsin NupTT 45 250 45 176 78°°
y
{
== =
^h
, Ndownwmg 30 98 294 {
== =
^^hh
e downward force of box on ground is:
,, NdownF 294 176 78 117 22
down
=- =
erefore:
, NupN 117 22 {
=
,, , Ntothe rightfN06 117 22 70 33
kk
{ {
n
== =
^^hh
(5)
Gr11_Physical_Science_Term1_Resource_Book.indb 24 2018/12/24 8:22:47 AM
25
WORKSHEETS
16.3
,
coscos NleftTT 45 250 45 176 78
°°
x
{
== =
^h
Fma
net
=
Tf ma
x
{
-=
,, a176 78 70 33 30
{
-=
^h
, a106 45 30
=
^h
,a 355
=
m·s
−2
to the le (5)
17. 17.1
Truck
F = 10000 N
T
PAGE 24 QUESTION 16.1 DIAGRAM
PAGE 25 QUESTION 17.1
PAGE 25 QUESTION 18.1
PAGE 26 QUESTION 20.2 DIAGRAM
cosTT 20°
x
{
=
Truck: Log:
Choose le as positive:
Fma
net
=
Fma
net
=
FT a2 000
x
{
-=
Ta
200
x
{
=
^h
cosTa10 000 20 2 000°
-=
cosTa20 200° {
=
(ii)
cosTa20 10 000 2 000° {
=-
(i)
Set equation (i) equal to equation (ii)
aa10 000 2 000 200
-=
a10 000 2 200
=
,a 455
=
m·s
−2
to the le (7)
17.2
cosTa20 200°
=
,
,
co
sN
N
T
T
20 200 455 910
968 40
°
`
{
{
==
=
^h
(2)
18. 18.1
PAGE 24 QUESTION 16.1 DIAGRAM
PAGE 25 QUESTION 17.1
PAGE 25 QUESTION 18.1
PAGE 26 QUESTION 20.2 DIAGRAM
, coscos Ntothe rightFF 40 300 40 229 81
°°
x
{{ {
== =
(3)
Gr11_Physical_Science_Term1_Resource_Book.indb 25 2018/12/24 8:22:55 AM
RESOURCE PACK
26
18.2 Choose right as positive:
Fma
net
=
Ff ma
x
{
-=
,(
)f2298
13
02
{
-=
^h
, f2298160
-=
, Ntothe leftf 169 81
{ {
=
(4)
18.3
, sinsin NdownFF 40 300 40 192 84°°
y
{
== =
,
Ndownwmg 30 98 294
{
== =
^^hh
,, NdownF 294 192 84 486 84
down
{ {
=+ =
(4)
19. Choose up as positive:
Fma
net
=
()Fw 2 000 20 {
-=
^h
F 49 000 40 000{
-=
NupF 89 000 { {
=
Upward Thrust F
Weight W
(4)
20. 20.1 3 kg: 5 kg:
Friction F = 10 N
Normal Force N
Tension T
Weight W
3 kg
(9)
20.2 3 kg:
,, sinsin Ndownslopeww30 39830147°°
x
{ {
== =
^^hh
5 kg:
,, sinsin Ndownslopeww30 59830245°°
x
{ {
== =
^^hh
30˚
w
(4)
20.3 3 kg: 5 kg:
Choose up the slope as positive:
Fma
net
=
Fma
net
=
()Tf 32
{
-=
^h
()FTf 52
{
--=
^h
T 10 6
-=
F 16 17 10
--=
NT 16 {
=
NF 43 {
=
(4)
21. When object A exerts a force on object B, object B simultaneously exerts an oppositely
directed force of equal magnitude on object A. (2)
Normal
force (N)
Friction f = 17 N
Gr11_Physical_Science_Term1_Resource_Book.indb 26 2018/12/24 8:23:08 AM

27
WORKSHEETS
22. 22.1 Action: Forward force of boy on car.
Reaction: Backward force of car on boy.
Action: Backward force of foot on road.
Reaction: Forward force of road on foot. (4)
22.2 Action: Downward force of Earth on light bulb.
Reaction: Upward force of light bulb on Earth.
Action: Downward force of light bulb on cord.
Reaction: Upward force of cord on light bulb. (4)
22.3 Action: Upward force of hand on ball.
Reaction: Downward force of ball on hand.
Action: Downward force of Earth on ball.
Reaction: Upward force of ball on Earth. (4)
22.4 Action: Forward force of donkey on cart.
Reaction: Backward force of cart on donkey.
Action: Backward force of hoof on road.
Reaction: Forward force of road on hoof. (4)
23. Every object in the universe attracts every other object in the universe with a force that
is directly proportional to the product of their masses and inversely proportional to
the square of the distance between their centres. (2)
24. 24.1
,
,,
,
Ntowards Mars
FG
r
mm
F
F
67 10
9 377 000
11 10 64 10
53610
2
12
11
2
16 23
15
#
##
#
{
{
{{
=
=
=
-
^
^
^
^
h
h
h
h
(4)
24.2
.
,
,
,
,ms
gG
M
g
g
67 10
11 110
11 10
59810
Phobos
Phobos
Phobos
Phobos
2
11
3
2
16
32
)
d
Phobos
#
#
#
#
{
{
{
=
=
=
-
--
^
^
^
^
h
h
h
(3)
24.3
gG
d
M
gG
d
M
G
d
M
G
d
M
g
2
1
10
1
4
1
10
1
10
1
1
4
5
2
Phobos
Deimos Phobos
2
2
2
2
#
{
{
{
=
==
==
a
a
k
k
Decrease by a factor of
5
2
(3)
Gr11_Physical_Science_Term1_Resource_Book.indb 27 2018/12/24 8:23:15 AM

RESOURCE PACK
28
25.
,
,
,N
FG
r
mm
F
F
67 10
15 10
6 500 610
116
2
12
11
9
2
24
#
#
#
{
{
{
=
=
=
-
^
^
^
^
h
h
h
h
(3)
26. 26.1
,
,
,.
gG
M
g
gm
s
67 10
3 380 10
64210
37710
Mars
Mars
Mars
Mars
2
11
3
2
23
32
)
d
Mars
#
#
#
#
{
{
{
=
=
=
-
--
^
^
^
^
h
h
h
(3)
26.2
,NWmg 500 3771885{ {
== =
^^hh
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 28 2018/12/24 8:23:16 AM
29
WORKSHEETS
MARKING GUIDELINES
CONSOLIDATION EXERCISE
TOTAL: 63 MARKS
Solutions:
1.1 e force that opposes the motion of an object and acts parallel to the surface the
object is in contact with. (2)
1.2
,, ,NfN04 3981176
max
ss
{ {
n
== =
^^^hh h
(2)
1.3
,,, Ntothe leftfN028398 823
kk
{{
n
== =
^^^hh h
(3)
1.4 3 kg: 2 kg:
Friction
F
Weight W
Normal N
Tension T
3 kg
Weight W
Tension T
2 kg
(6)
1.5 When a net force, F
net
, is applied to an object of mass, m, it accelerates in the
direction of the net force. e acceleration, a, is directly proportional to the net
force and inversely proportional to the mass. (3)
1.6 3 kg: 2 kg:
Choose right as positive: Choose down as positive:
Fma
net
=
Fma
net
=
Tf a3 {
-=
^h
wT a2 {
-=
^h
,Ta8233
-=
, Ta19 62
-=
,Ta8234{
=+
(i)
,Ta19 62{
=-
(ii)
Set equation (i) equal to equation (ii)
,,aa8234 19 62
+= -
,a61137
=
,a 190
=
m·s
−2
to the right and downwards (6)
One mark for each
correct force which is
also labelled correctly.
Gr11_Physical_Science_Term1_Resource_Book.indb 29 2018/12/24 8:23:37 AM
RESOURCE PACK
30
2.1
1 mark for each correctly labelled force
Friction F
Weight W
Normal N
(3)
2.2
60˚
w
,, sinsin Ndownthe slopeww60 1 000 98 60 8 489 05
x
cc
{{
== =
^^hh
,
coscos Nperpendicular to theslopeww 60 1 000 98 60 4 900
y
#
cc
{{
== =
^h
(4)
2.3
,
Nupthe slopefN03 4 900 1 470
{{ {
n
== =
^^hh
(3)
2.4 If mass increases, weight w increases; w
y
increases, N increases.
erefore friction increases. (3)
2.5
,, Ndownthe slopeFwf 8 487 05 1 470 7 017 05
netx
{{{
=-=-=
(3)
2.6 e net force will decrease because w
x
decreases (only if we assume friction to
be constant). (2)
2.7
Fma
net
=
,()a7 017 05 1 000
{{
=
,a 702
=
m·s
−2
down the slope (4)
3.1
Weight W
Tension Q
Y
Tension P
(3)
3.2 Yes Point Y remains at rest therefore the net force acting on point Y is zero (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 30 2018/12/24 8:23:47 AM
WORKSHEETS
3.3 Triangle of forces method:
600 N
Q
w
90˚
60˚
tan
Q
30
600
°
{
=
,tanNQ 600 30 346 41° {
==
(4)
OR Components method:
P = 600 N
w
Q
30˚
30˚
60˚
coscos NPP 60 600 60 300°°
x
{ {
== =
^h
cosPQ Q 30°
xx
{
==
cosQ300 30°
=
,NQ 346 41 {
=
(4)
3.4 Triangle of forces:
cos
w
30
600
°
{
=
,
cos
Nw
30
600
692 82
°
{
==
,
,
,k
gm
g
w
98
692 82
70 70
{
{
== =
(4)
OR Components method:
,sinsin NPP 60 600 60 519 62
°°
y
{
== =
^h
,,sinsin NQQ30 346 41 30 173 21°°
y
{
== =
^h
,,
,N
wPQ 519 62 173 21 692 83
yy
{
=+ =+=
,
,
,k
gm
g
w
98
692 83
70 70
{
== =
(4)
Gr11_Physical_Science_Term1_Resource_Book.indb 31 2018/12/24 8:24:20 AM

RESOURCE PACK
32
4.1
gG
d
M
2
=
,
,
,
,k
gM
G
gd
67 10
75
13 10
18910
2
11
8
2
27
#
#
#
{{
{
== =
-
^
^
^
h
h
h
(3)
4.2
gG
d
M
A
A
J
2
=
GM gd
JA
A
2
=
gG
d
M
M
M
J
2
=
GM gd
JM
M
2
=
GM gd gd
JAAMM
22
{
==
,
,d
g
gd
1
34 090
13 10
57610
M
M
AA
2
2
8
2
20
#
#
#
{
== =
^h
,d 57610
M
220
#
=
,md 24010
M
10
`
#
{
=
(3)
Gr11_Physical_Science_Term1_Resource_Book.indb 32 2018/12/24 8:24:32 AM

33
WORKSHEETS
TOPIC 3: Atomic Combinations
(Molecular Structure)
WORKSHEET
MULTIPLE CHOICE
1. e type of particle which results from the covalent bonding of atoms is called:
A an ion.
B an atom.
C a molecule.
D an isotope. (2)
2. e bond in the H
2
O molecule is described as polar covalent because :
A the shared electrons are closer to the oxygen atom than the hydrogen atom.
B the shared electrons are closer to the hydrogen atom than the oxygen atom.
C there is a large electronegativity dierence between the two atoms.
D electrons are transferred from hydrogen atoms to oxygen atoms. (2)
3. e CCl
4
molecule is considered to be non-polar because :
A the shape of the molecule is linear.
B the electronegativity dierence is very small.
C the molecule is asymmetrical.
D the molecule is symmetrical. (2)
4. Consider the methane molecule (CH
4
). How much energy is required to break the
chemical bonds in the molecule (in kJmol
−1
)?
A 412
B 348
C 1648
D 1996
(2)
BOND BOND ENERGY
C – C 348
C = C 619
C = C 835
C - H 412
C = O 799
O - H 463
O = O 499
Gr11_Physical_Science_Term1_Resource_Book.indb 33 2018/12/24 8:24:32 AM
RESOURCE PACK
34
LONG QUESTIONS
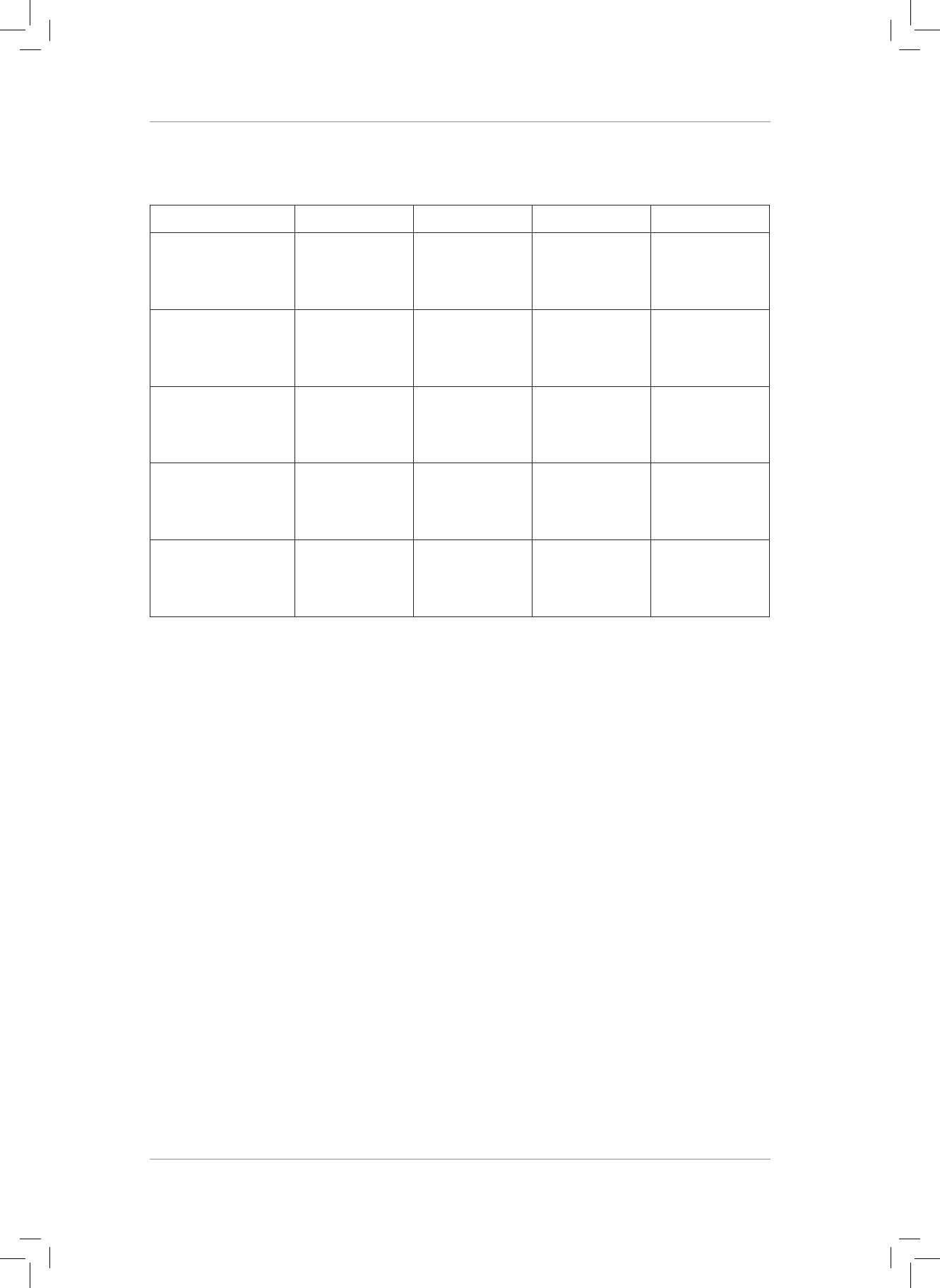
1. Copy and complete the following table:
MOLECULE CO
2
CH
4
SF
6
NH
3
What is the central
atom in the
molecule?
Shape of molecule
Are the molecular
shapes IDEAL or
NON-IDEAL?
Does the molecule
contain polar bonds?
Is the molecule polar
or non-polar?
(20)
2. A water molecule is polar while a carbon dioxide molecule is non-polar.
2.1 What is the dierence between a polar and a non-polar molecule? (4)
2.2 Use electronegativity dierence to determine the type of bond present in the
carbon dioxide molecule. (2)
2.3 State the shapes of water and carbon dioxide molecules. (2)
2.4 Explain why carbon dioxide, which contains polar bonds, is non-polar. (2)
3. What is the nature of the charges on the H and O atoms in the H
2
O molecule?
Explain your answer. (2)
4. H
2
O can form a dative covalent bond to form H
3
O
+
. Use the Lewis diagram for a water
molecule to help explain the idea of a dative covalent bond. (6)
Gr11_Physical_Science_Term1_Resource_Book.indb 34 2018/12/24 8:24:32 AM

35
WORKSHEETS
5. Consider the following list of molecules:
Cl
2
H
2
O BF
3
HCl CO
2
NH
3
5.1 Which of the following molecules is polar covalent and which are non-polar
covalent? (6)
5.2 CO
2
contains polar bonds, but is considered to be a non-polar molecule.
Explain why this is so. (3)
5.3 Name the shape of each molecule in the list. (7)
6. Consider a molecule of hydrogen sulde (H
2
S).
6.1 Draw the Lewis diagram for hydrogen sulde. (2)
6.2 Identify the central atom in H
2
S. (1)
6.3 How many lone pairs of electrons are present in H
2
S molecule? (1)
6.4 How many bonding pairs of electrons are present in the H
2
S molecule. (1)
6.5 What is the molecular shape of this molecule? (1)
6.6 Is this an IDEAL or a NON-IDEAL molecular shape? Explain your answer. (3)
7. e table below lists the bond energies of certain covalent bonds.
BOND O = O O-O C - C C = O C - H H - H H - O
BOND ENERGY
(kJmol
–1
)
494 142 346 499 413 435 459
7.1
Use the information from the table to compare the length and strength of an
O – O bond to the length and strength of an O = O bond. (3)
Consider the chemical equation shown below:
2C
2
H
2
+ 7O
2
$ 4CO
2
+ 6H
2
O
7.2 Calculate the total energy absorbed to break the bonds of the reactants. (5)
7.3 Calculate the total energy released on bond formation of the products. (4)
7.4 How much excess energy was released in the whole reaction? (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 35 2018/12/24 8:24:33 AM
RESOURCE PACK
36
CONSOLIDATION EXERCISE
MULTIPLE CHOICE
1. A covalent bond will form between two atoms when...
A there is repulsion between the nuclei of two approaching atoms.
B there is attraction between the electrons of two approaching atoms.
C one atom transfers an electron to another atom.
D the potential energy of the atoms is at its lowest. (2)
2. In VSEPR theory, the greatest amount of repulsion between orbitals will be between:
A lone pair and lone pair.
B bonding pair and lone pair.
C bonding pair and bonding pair.
D bonding pair and central atom. (2)
3. e HBr molecule forms a dipole because the...
(i) Br atom is more electronegative than the H atom.
(ii) H atom is more electronegative than the Br atom.
(iii) HBr molecule is polar.
Which of the above statements is/are true?
A Only (i) is true.
B Both (i) and (iii) are true.
C Both (ii) and (iii) are true.
D Only (ii) is true. (2)
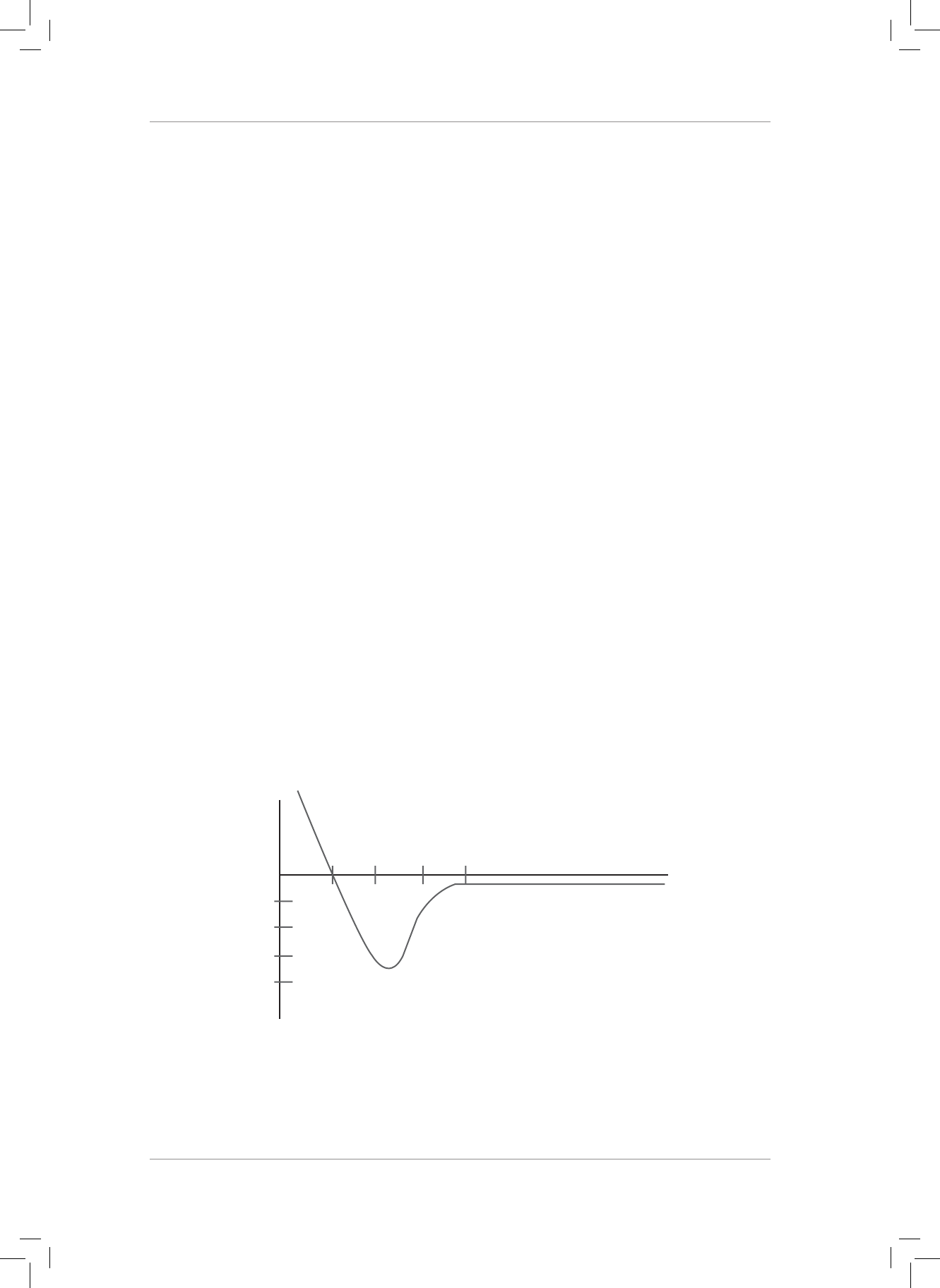
Study the energy diagram below which represents the formation of a covalent bond
between two atoms. Use this diagram to answer Questions 4 and 5.
Potential energy
of the system
(kJ)
Bond length (nm)
0,100
-100
-200
-300
-400
0,200 0,300 0,400
Gr11_Physical_Science_Term1_Resource_Book.indb 36 2018/12/24 8:24:33 AM

37
WORKSHEETS
4. e bond energy of the of the chemical bond is ...
A 0 kJ
B 30 kJ
C 330 kJ
D 200 kJ (2)
5. e bond length between the two atoms forming the chemical bond is ...
A 0,200 nm
B 0,370 nm
C 0,240 nm
D 0,050 nm (2)
6. Using the table alongside, calculate the total
energy required to break the bonds of the
reactants when methane reacts with oxygen to
form carbon dioxide and water. e reaction
equation is written below.
CH
4
+ 2O
2
$ CO
2
+ 2H
2
O
BOND BOND ENERGY
(kJmol
–1
)
C – C 348
C = C 619
C = C 835
C - H 412
C = O 799
O - H 463
O = O 499
A 911 kJmol
-1
B 1 410 kJmol
-1
C 2 646 kJmol
-1
D 3 644 kJmol
-1
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 37 2018/12/24 8:24:33 AM

RESOURCE PACK
38
LONG QUESTIONS
1. Consider the table below. Redraw this table in your answer books and complete it in the
spaces provided.
CHEMICAL
FORMULA OF
COMPOUND
ELECTRO-
NEGATIVITY
DIFFERENCE
NON-POLAR
COVALENT,
POLAR
COVALENT OR
IONIC BOND
SHAPE OF
MOLECULE
TYPE OF
MOLECULE
(POLAR OR
NON-POLAR)
2
4
CO
2
3
H
2
O
3
(28)
2. Draw Lewis structures for:
2.1 a water molecule.
2.2 a carbon dioxide molecule. (4)
3. 3.1 Dene the term electronegativity. (2)
3.2 Consider the following molecules:
F
2
HCl CH
4
H
2
O NH
3
SF
6
a) Assign electronegativity values to each atom. (6)
b) Calculate the electronegativity dierence for the bonds ineach molecule. (6)
c) Identify which of the molecules contain polar covalent or non-polar covalent
bonds. (6)
d) Identify the shapes of each molecule using VSEPR theory. (6)
4. Consider the following list of molecules:
HF BCl
3
CF
4
NH
3
CO
2
SCl
6
4.1 Draw Lewis diagrams for the molecules HF and NH
3
to show the bonding that
takes place. (4)
4.2 Which of the molecules in the list are non-polar? Explain your answer. (6)
4.3 According to VSEPR theory, what are the shapes of molecules
BCl
3
, CF
4
, NH
3
and SCl
6
? (4)
Gr11_Physical_Science_Term1_Resource_Book.indb 38 2018/12/24 8:24:33 AM
39
WORKSHEETS
4.4 NH
3
is said to have a non-ideal shape where SCl
6
is said to have an ideal
shape. What is the dierence between molecules with these dierent shape
classications? (2)
5. For each of the following bonding pairs, say which bond is more polar. Show all
calculations and indicate the partially positive (δ
+
) and partially negative (δ
–
) poles on
each bond.
5.1 C – O and C – N (3)
5.2 P – Br and P – Cl (3)
5.3 C – O and C - S (3)
6. Ammonia (NH
3
) is able to make a dative covalent bond with a hydrogen ion (H
+
)
forming the ammonium cation (NH
4
+
)
6.1 Draw the Lewis diagram for a molecule of ammonia. (2)
6.2 Explain why the hydrogen ion can form a dative covalent bond with ammonia. (2)
6.3 Draw a Lewis diagram of the resulting ammonium cation. (3)
7. 7.1 Dene the term ‘bond energy’. (2)
7.2 What is the relationship between bond energy and bond length in a
stable molecule? (2)
7.3 Consider the table of bond energies and bond lengths below. Use the table to
answer the following questions
a) Cl
2
and I
2
are both examples of diatomic
halogen molecules (Group 17). Explain
why I
2
has a smaller bond energy than Cl
2
.
(2)
b) Explain why I
2
has a larger bond length
compared to Cl
2
. (2)
c) Explain why the bond energies of the
dierent carbon to carbon bonds dier
from single to triple bonds. (3)
BOND BOND
ENERGY
(kJ.mol
–1
)
BOND
LENGTH
(pm)
242 199
C - C 347 540
C = C 614 340
C = C 839 120
I - I 151 266
Gr11_Physical_Science_Term1_Resource_Book.indb 39 2018/12/24 8:24:34 AM

RESOURCE PACK
40
8. Consider the reaction between oxygen and hydrogen which is used to form water:
O
2
+ 2H
2
$ 2 H
2
O
BOND BOND ENERGY (kJ.mol
–1
)
O=O 498
O - H 463
H - H 436
Using the table of bond energies supplied, calculate the following quantities.
8.1 e amount of energy required to break the bonds in the reactants. (3)
8.2 e amount of energy released on formation of bonds of products. (2)
8.3 e total amount of energy released from the reaction in the formation of H
2
O. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 40 2018/12/24 8:24:34 AM
WORKSHEETS
MARKING GUIDELINES
MULTIPLE CHOICE
1.1 C All molecules are made from non-metal atoms only in their structure.
1.2 A e electronegativity of O is greater than that of H, thus the shared electron pair
will be closer to the O atom and result in the bond becoming polar. Water is
an asymmetric molecule and will thus will have a denite δ
+
and δ
−
end, hence
polar covalent.
1.3 D e central C atom is surrounded by 4 Cl atoms to form a tetrahedral shape.
Tetrahedrons are symmetrical arrangements hence the molecule will be non-
polar covalent due to symmetry.
1.4 C ere are 4 × C – H bonds in the molecule, thus it is simply 4 × 416 kJmol
−1
to
give a total amount of energy to break the bonds as 1 648 kJmol
−1
(8)
LONG QUESTIONS
1. Complete the following table
MOLECULE CO
2
CH
4
SF
6
NH
3
WHAT IS THE
CENTRAL ATOM IN
THE MOLECULE?
C
C S
SHAPE OF MOLECULE
ARE THE
MOLECULAR SHAPES
IDEAL OR NON-
IDEAL?
DOES THE MOLECULE
CONTAIN POLAR
BONDS?
IS THE MOLECULE
POLAR OR NON-
POLAR?
(20)
2. 2.1 A polar molecule – A molecule with a distinct region of charge at either end of the
molecule due to one atom having a larger electronegativity than the other.
A non-polar molecule – ere is no distinct region of charge due to a zero
electronegativity dierence between the atoms or the molecule is perfectly
symmetrical. (4)
2.2 C : 2,5 electronegativity dierence is 1,0 thus bonds are
O : 3,5
}
polar covalent (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 41 2018/12/24 8:24:34 AM
RESOURCE PACK
42
2.3 water – angular
carbon dioxide – linear (2)
δ
–
δ
+
δ
–
2.4 Carbon dioxide is non-polar due to the symmetry of the molecule. O = C = O
ere is no distinct region of δ
+
and δ
–
, thus the molecule is considered non-polar
even though it has polar bonds. (2)
3. In the water molecule, H has a δ
+
charge and O has a δ
–
charge. is is due to the
electronegativity of O being greater than the electronegativity of H. (2)
4. H
2
O has two lone pairs of electrons. One of these lone pairs can be used to ll the
empty outermost energy level of the H
+
ion. is now forms a dative covalent bond
between H
2
O and H
+
producing the H
3
O
+
ion.
(for correct diagram)
H
+
H
H
+
+
O
x
x
H
H
O
x
x
(6)
5. 5.1 Polar covalent : H
2
O HCl NH
3
Non-polar covalent : Cl
2
BF
3
CO
2
(6)
5.2 CO
2
is a symmetrical molecule meaning that it does not have two denite
regions of polarity , that is, a δ+ side and a δ- side. Even though the bonds are
polar due to the electronegativity dierence between C and O, there is no distinct
polarity within the molecule, hence it is non-polar.
δ
–
δ
+
δ
–
O = C = O (3)
5.3 Cl
2
: linear
H
2
O: angular
BF
3
: trigonal planar
HCl: linear
CO
2
: linear
NH
3
: trigonal pyramidal (6)
Gr11_Physical_Science_Term1_Resource_Book.indb 42 2018/12/24 8:24:34 AM
43
WORKSHEETS
6. 6.1
correct atoms and shape
S
x
x
HH
correct electron arrangement (2)
6.2 S (1)
6.3 two (1)
6.4 two (1)
6.5 angular (1)
6.6 NON-IDEAL - the central atom has lone pairs of electrons around it. (3)
7. 7.1 e O = O bond requires more energy to break the bond compared to the
O – O bond.
e greater the amount of energy required, the shorter the bond length,
thus the O = O bond will be shorter in length in comparison to the O – O bond.
(3)
7.2
HH
HH
HHCC+7 x O = O4 x O = C = O + 6 xO
H H
2 × ( C – C ) = 692 + 7 × ( O = O) = 3 458
2 × ( 6 × C – H ) = 4 956
Total energy to break bonds of reactants = 5 648 + 3 458
Total = 9 106 kJmol
−1
(5)
7.3 4 × (2 × C = O) = 3 992 kJmol
−1
+ 6 × ( 2 × O – H ) = 5 508 kJmol
−1
Total energy released on bond formation of products = 3 992 + 5 508
Total = 9 500 kJmol
−1
(4)
7.4 Excess energy = (energy to break bonds) - (energy released in new bond formation)
= 9 106 − 9 500
= −394 kJmol
−1
us, 394 kJmol
−1
of excess energy was released (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 43 2018/12/24 8:24:36 AM
RESOURCE PACK
44
MARKING GUIDELINES
CONSOLIDATION EXERCISE
TOTAL : 122 MARKS
MULTIPLE CHOICE
1. D When atoms approach each other, there are both attractive and repulsive
forces between the atoms. Only when these forces are balanced will a covalent
bond be formed and the potential energy of the system will be at its lowest.
(2)
2. A Lone pair-lone pair electron repulsions are the largest of all the valance
electron pair repulsions. (2)
3. B Br has an electronegativity of 2,8 and H has an electronegativity of 2,1. is
gives an electronegativity dierence of 0,7 which makes the bond polar. HBr
is also a linear molecule which will form denite δ+ and δ- ends as a result of
this dierence, thus HBr will be polar. (2)
4. C e bond energy will be at the position where the potential energy in the
system is the lowest and the bond is stable. is is represented by the lowest
point of the graph and the value is read o the y-axis of the graph. (2)
5. C At the point where the potential energy in the system is the lowest is where
the bond is most stable. is is where the atoms now establish a stable
chemical bond and the bond length is xed. is is now read o the x – axis
of the graph. (2)
6. C is is the sum of the bond energies of four C – H bonds and two O = O
bonds as there are 2 moles of O
2
in the reactants. is adds up to 2 646
kJmol
−1
. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 44 2018/12/24 8:24:37 AM
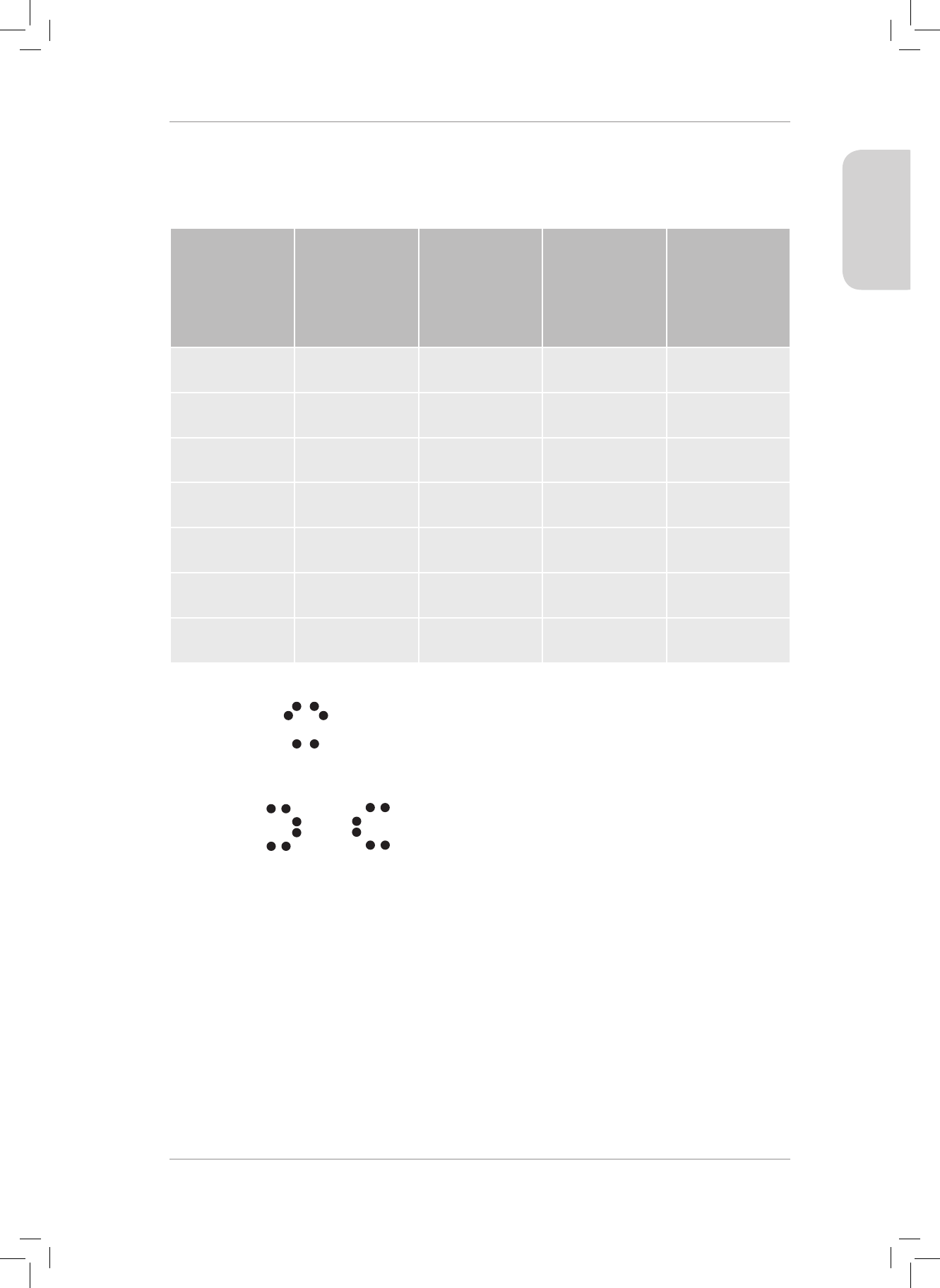
45
WORKSHEETS
xx
x
x
x
HH
O
O
x
x
C
O
LONG QUESTIONS
1. Consider the table below. Redraw this table in your answer books and complete in the
space provided.
CHEMICAL
FORMULA OF
COMPOUND
ELECTRO-
NEGATIVITY
DIFFERENCE
BETWEEN
ATOMS IN
BOND
NON-POLAR
COVALENT,
POLAR
COVALENT OR
IONIC BOND
SHAPE OF
MOLECULE
IDEAL OR NON
IDEAL SHAPE
Cl
2
0
Non-polar
covalent
Linear IDEAL
CF
4
1,5
Non-polar
covalent
Tetrahedral IDEAL
CO
2
1,0
Non-polar
covalent
Linear IDEAL
HBr
0,7
Polar covalent
Linear IDEAL
NH
3
0,9
Polar covalent
Pyramidal NON-IDEAL
H
2
O
1,4
Polar covalent
Angular NON-IDEAL
BF
3
2,0
Non-polar
covalent
Trigonal planar
IDEAL
(28)
2. 2.1
correct shape
electrons arranged correctly (2)
2.2
correct shape
electrons arranged correctly (2)
3. 3.1 is is the measure of the amount of attraction that an atom exerts on a shared
electron pair within a chemical bond. (2)
3.2 a) F
2
: F = 4,0 HCl : H = 2,1 CH
4
: C = 2,5
Cl = 3,0 H = 2,1
H
2
O : H = 2,1 NH
3
: N = 3,0 SF
6
: S = 2,5 (6)
O = 3,5 H = 2,1 F = 4,0
Gr11_Physical_Science_Term1_Resource_Book.indb 45 2018/12/24 8:24:37 AM

RESOURCE PACK
46
b) F
2
: EN dierence 4,0 – 4,0 = 0
HCl: EN dierence 3,0 – 2,1 = 0,9
CH
4
: EN dierence 2,5 – 2,1 = 0,4
H
2
O: EN dierence 3,5 – 2,1 = 1,4
NH
3
: EN dierence 3,0 – 2,1 = 0,9
SF
6
: EN dierence 4,0 – 2,5 = 1,5 (6)
c) F
2
: non-polar covalent
HCl: polar covalent
CH
4
: polar covalent
H
2
O: polar covalent
NH
3
: polar covalent
SF
6
: polar covalent (6)
d) F
2
= linear
HCl = linear
CH
4
= tetrahedral
H
2
O = angular
NH
3
= trigonal pyramidal
SF
6
= octahedral (6)
4. 4.1
x
x
x
HH
x
H
NF
Two marks for each correct structure.
(4)
4.2 BCl
3
CF
4
CO
2
SCl
6
symmetrical molecules hence non-polar
(6)
4.3 BCl
3
: trigonal planar
CF
4
: tetrahedral
NH
3
: trigonal pyramidal
SCl
6
: octahedral (4)
4.4 NH
3
- has lone pair of electrons around central atom hence NON-IDEAL
SCl
6
- has no lone pairs of electrons around central atom, hence IDEAL (2)
5. 5.1 δ
+
δ
−
C – O EN dierence (C – O) = 3,5 – 2,5 = 1,0
C – N EN dierence ( C – N) = 3,0 – 2,5 = 0,5 (3)
5.2 δ
+
δ
−
P – Cl EN dierence (P – Cl) = 3,0 – 2,1 = 0,9
P – Br EN dierence (P – Br) = 2,8 – 2.1 = 0,7 (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 46 2018/12/24 8:24:38 AM

47
WORKSHEETS
5.3 δ
+
δ
−
C – O EN di erence ( C - O) = 3,5 – 2,5 = 1,0
C – S EN di erence (C – S ) = 2,5 – 2,5 = 0 (3)
6.1
PAGE 43 6.1 DIAGRAM
PAGE 45 QUESTION 2.1
PAGE 45 QUESTION 2.2
PAGE 46 QUESTION 4.1
PAGE 47 QUESTION 6.1
PAGE 47 QUESTION 6.3
(2)
6.2 N has a lone pair of electrons in the ammonia molecule which it can share with the
H
+
ion which has an empty valence energy level.
(2)
6.3 for the correct Lewis diagram
ere is now an arrow replacing the
lone pair of electrons to
PAGE 43 6.1 DIAGRAM
PAGE 45 QUESTION 2.1
PAGE 45 QUESTION 2.2
PAGE 46 QUESTION 4.1
PAGE 47 QUESTION 6.1
PAGE 47 QUESTION 6.3
indicate dative covalent bond (3)
7.1 e bond energy of a compound is the energy needed to break one mole of its
molecules into separate atoms. (2)
7.2 As the bond length gets larger, so the amount of energy required to break the bond
gets smaller.
(2)
7.3 a) I
2
is made up of much larger atoms than in Cl
2
and thus has a much greater
bond length. e larger the bond length, the less the bond energy. (2)
b) I
2
is made up of much larger atoms compared to Cl
2
. e larger the atoms,
the larger the bond length between the atoms. (2)
c) e more bonds there are between two atoms, the shorter the bond length.
is means that the amount of energy required to break the bonds will be
more, thus bond energy will increase. e carbon triple bond required
839kJmol
-1
of energy to break the bonds while the carbon single bond only
requires 347kJmol
-1
to break the bond. (3)
8.1 O = O 498 kJmol
-1
2 × H – H 872 kJmol
-1
Total 1 370 kJmol
-1
(3)
8.2 4 × O – H : 1 852 kJmol
-1
(2)
8.3 Energy released = 1 370 – 1 852
= –482 kJmol
-1
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 47 2018/12/24 8:24:38 AM

RESOURCE PACK
48
TOPIC 4: Intermolecular Forces and
Chemistry of Water
WORKSHEET
MULTIPLE CHOICE
1. Which one of the following statements represents the best explanation for the term
‘electronegativity’?
A A measure of the tendency of an atom to attract a bonding pair of electrons.
B A measure of the tendency of an atom to attract an electron.
C A measure of the strength of a covalent bond.
D A measure of the strength of an ionic bond. (2)
2. Sodium chloride (NaC) is a solid which is soluble in water. Which one of the following
describes the intermolecular forces that exist between sodium chloride and water in
solution?
A Ion-dipole
B Dipole-dipole
C Ion-induced dipole
D Induced dipole-dipole (2)
3. Hydrogen bonding is a type of intermolecular force that can exist between the
molecules of certain compounds. Which one of the statements below best describes the
conditions under which hydrogen bonding is most likely to occur? It occurs between:
A small molecules which contain hydrogen atoms.
B molecules in which hydrogen is bonded to small atoms with high
electronegativity.
C large molecules which contain both hydrogen and oxygen atoms.
D molecules in which hydrogen is bonded to small atoms with low
electronegativity. (2)
4. Consider the structure of hexane.
H
HC
H
H
C
H
H
C
H
H
C
H
H
C
H
H
HC
H
A molecule of hexane is considered to be non-polar. Which one of the following
statements best describes the reason why hexane is non-polar?
Gr11_Physical_Science_Term1_Resource_Book.indb 48 2018/12/24 8:24:39 AM

49
WORKSHEETS
A Hexane contains only single bonds between atoms.
B e electronegativity dierence between C and H atoms is so small as to be
considered non-polar.
C Hexane is a linear molecule hence is symmetrical.
D e charge distribution of electrons within the hexane molecule is symmetrical.
(2)
LONG QUESTIONS
1. Calcium chloride is prepared according to the following balanced chemical equation:
Ca(s) + 2HCl(aq) $ CaCl
2
(aq) + H
2
(g)
Hydrochloric acid is produced for this preparation by the ionisation of hydrogen
chloride gas in water.
1.1 Name the type of bonding that is present in a molecule of hydrogen chloride. (1)
1.2 Dene the term ‘electronegativity’. (2)
1.3 Make use of the Pauling scale of electronegativities, as provided in the Periodic
Table, to explain the type of bonding found in hydrogen chloride and crystalline
calcium chloride. (4)
1.4 HCl molecules are described as ‘dipoles’. Explain what is meant by this term. (2)
1.5 Calcium chloride is soluble in water. e structure of its crystal lattice is broken
down by the water molecules to form aqueous ions in solution.
1.5.1 Name the type of crystal lattice of which calcium chloride is an example. (1)
1.5.2 Name the types of intermolecular forces present between:
a) calcium chloride ions in the crystal lattice. (1)
b) the water molecules of the solvent. (1)
c) the water and the crystal lattice. (1)
1.5.3 Using diagrams to illustrate your answer, explain how the crystal lattice of
calcium chloride is broken down during the dissolving process. (5)
2. Use only substances from the list below when answering Question 2.1 to 2.6 (Only
write down the question number and the formula of the substance next to it.) e state
symbols (phase indicators) represent the physical state of each of the substances at room
temperature.
SiO
2
(s) HC(g) H
2
O() PH
3
(g) Mg(s) Br
2
(g) KF(s)
Select one substance from the list that has:
2.1. pure covalent intramolecular bonds. (1)
2.2 a high melting point due to the strong electrostatic attraction between the cations
and anions in the crystal lattice. (1)
2.3 hydrogen bonding intermolecular forces. (1)
Gr11_Physical_Science_Term1_Resource_Book.indb 49 2018/12/24 8:24:39 AM

RESOURCE PACK
50
2.4 a very high melting point due to its giant covalent network structure. (1)
2.5 delocalised valence electrons. (1)
2.6 dipole – dipole intermolecular forces. (1)
3. Consider the Table below which shows the boiling points of the halogens.
Halogens
2
CI
2
2
I
2
Boiling point (
o
C) -188 -35 59 184
3.1
Name the specic type of interatomic bond that occurs between atoms in the F
2
molecule. (2)
3.2 e table show an increase in the boiling points of the halogens from F
2
to I
2
.
Explain this trend by making reference to the relevant intermolecular force
between the halogen molecules and the factor inuencing its strength. (4)
4. Consider the following pairs of substances.
HCl and CO NaCl and CCl
4
KBr and H
2
S CCl
4
and Br
2
Which of the pairs will have:
4.1 ion-dipole intermolecular forces? (2)
4.2 dipole – dipole intermolecular forces? (2)
4.3 ion – induced dipole intermolecular forces? (2)
4.4 induced dipole – induced dipole intermolecular forces? (2)
5. Water is considered to be a highly remarkable substance that has many properties that
are benecial to life forms on earth. One of the interesting factors with regards to this is
the fact that ice is able to oat on water.
5.1 Explain why ice is able to oat on water. (2)
5.2 Why is this benecial to aquatic life? (1)
Gr11_Physical_Science_Term1_Resource_Book.indb 50 2018/12/24 8:24:39 AM
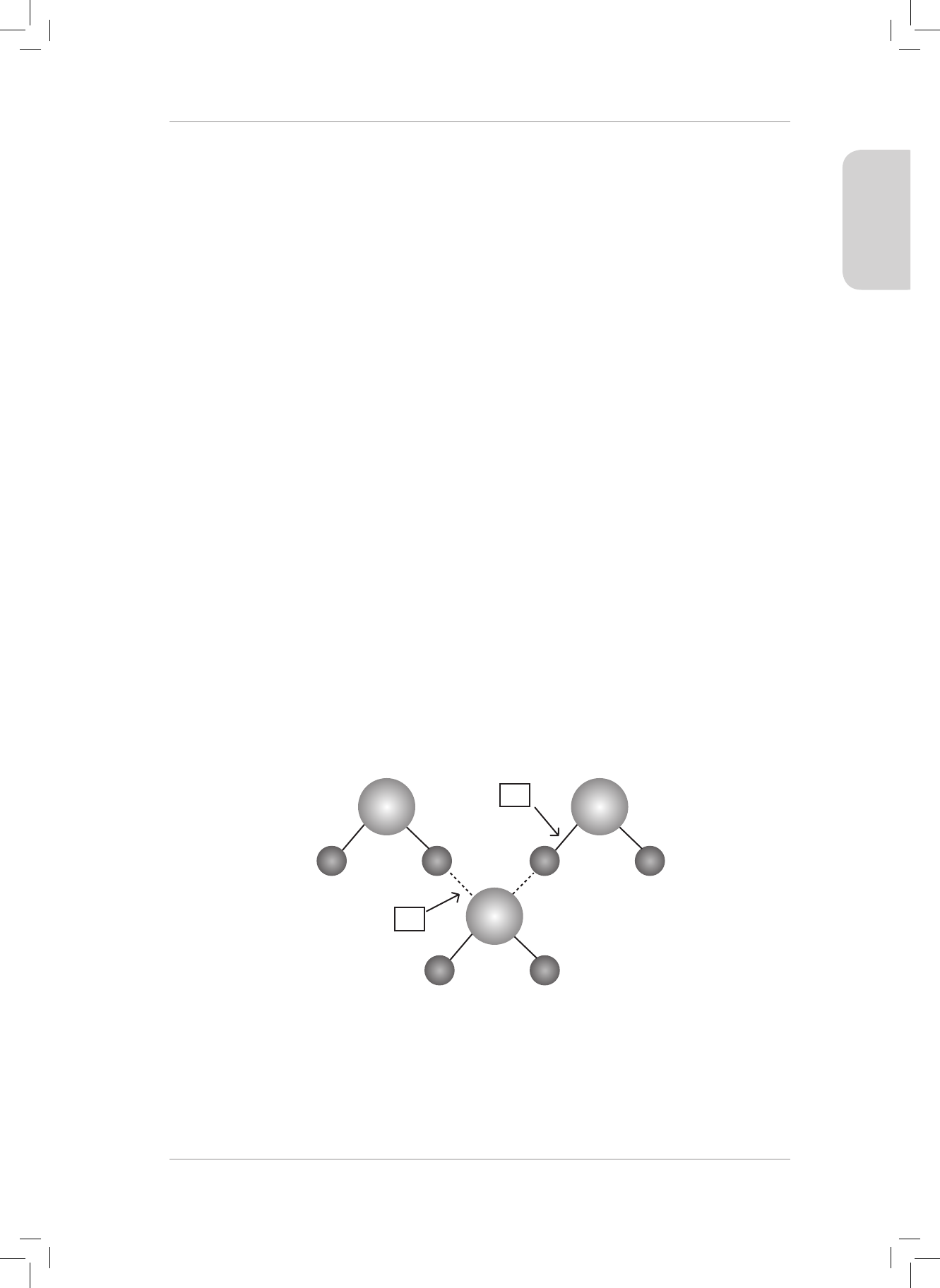
WORKSHEETS
CONSOLIDATION EXERCISE
TOTAL: 47 MARKS
1. Hydrogen uoride (HF) can be prepared by treating calcium uoride (CaF) with an
acid.
1.1 Dene the term ‘electronegativity’. (2)
1.2 Use electronegativity dierences to explain the dierence in the type of bonding
found in HF and CaF
2
. (4)
1.3 Using a Lewis diagram, illustrate fully how bonding occurs in CaF
2
. (4)
1.4 HF is described as a ‘polar molecule’. Explain what is meant by this term. (2)
1.5 In fact, HF is an example of a group of three molecules which exhibit what is
known as hydrogen bonding. What TWO identifying characteristics are crucial in
a molecule being able to exhibit hydrogen bonding? (2)
2. Two crystalline solids, P and Q have melting points of 710
o
C and 723
o
C respectively. At
700
o
C, substance P conducts electricity but Q does not. At 750
o
C, both P and Q conduct
electricity.
2.1 What can be deduced from the melting points about the relative magnitude of the
forces between the particles in P and Q? (2)
2.2 Explain the dierence observed in electrical conductivity between P and Q. (4)
2.3 You are told that one of the solids is barium and the other is potassium iodide.
Which is P and which is Q? (2)
3. Consider the diagram below, showing an arrangement of water molecules in the liquid
phase.
O
H H
O
H H
O
H H
Y
X
3.1 Explain the term ‘interatomic bond’. (1)
3.2 Name the specic type of interatomic bond represented by the letter X in the
diagram. (2)
3.3 Dene the term ‘intermolecular force’. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 51 2018/12/24 8:24:39 AM
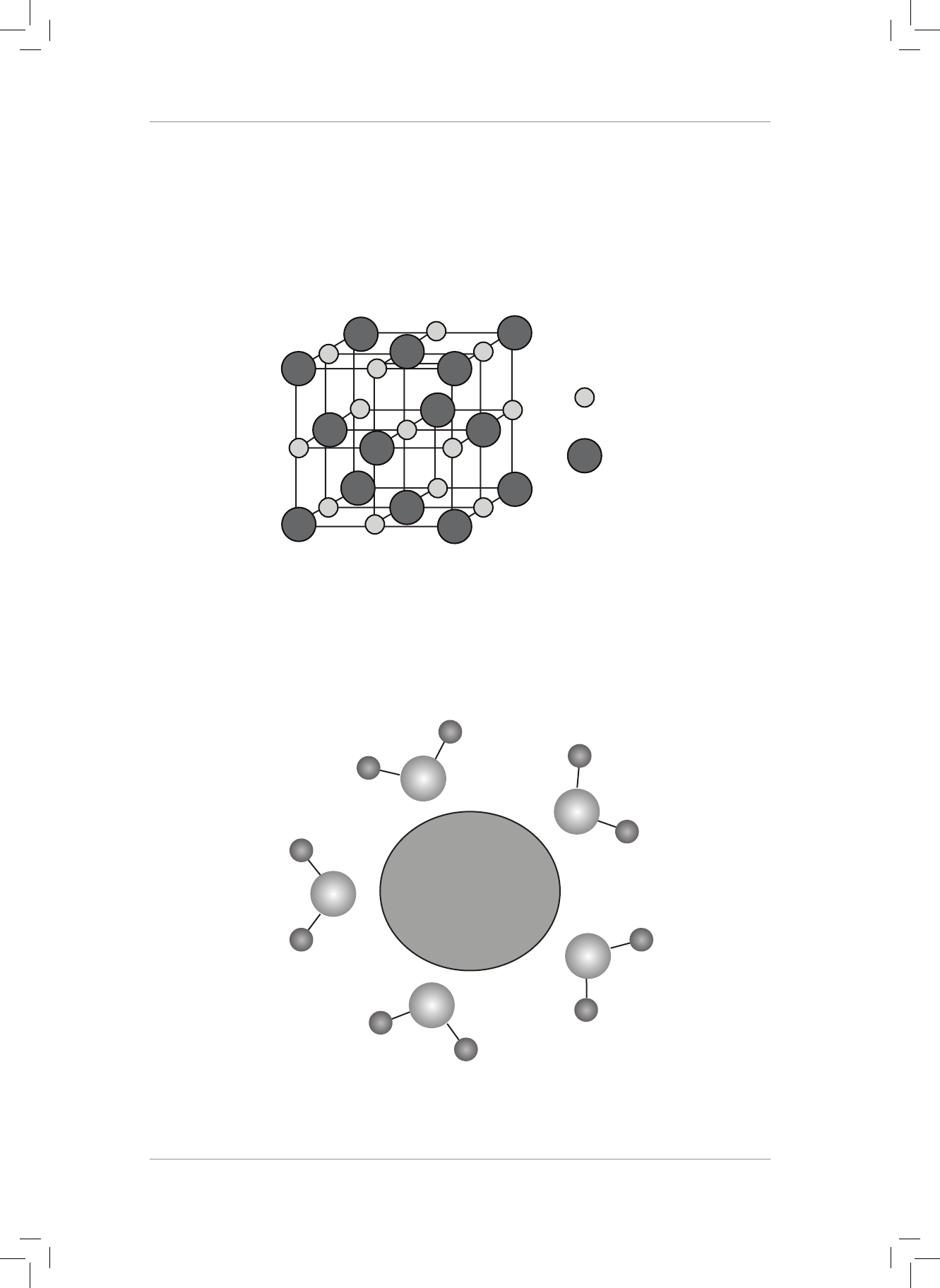
RESOURCE PACK
52
3.4 Name the specic type of intermolecular force represented by the letter Y in the
diagram. (1)
3.5 State two properties of the oxygen atom that make this type of intermolecular force
(Y) possible. (2)
3.6 What is the partial charge (δ
+
or δ
–
) on the hydrogen atom in a water molecule? (1)
4. Diagram 1 below shows the crystal lattice structure of the giant ionic solid sodium
chloride.
Diagram 1
+
Cl
Na
-
4.1 Dene the term ‘ionic bond’. (2)
4.2 With reference to the crystal lattice structure, explain why sodium chloride has a
very high melting point (801 °C). (3)
4.3 Sodium chloride dissolves in water. e ions are surrounded by water molecules as
shown in Diagram 2 below.
ION
Diagram 2
Gr11_Physical_Science_Term1_Resource_Book.indb 52 2018/12/24 8:24:40 AM

53
WORKSHEETS
a) Name the specic type of force between the ion and the water molecules. (1)
b) Is the ion shown in the above diagram a sodium ion (Na
+
) or a chloride ion
(C
–
)? Explain. (2)
c) A relatively large amount of water is needed to dissolve a small amount of
sodium chloride. Explain why this is so by referring to the structure of sodium
chloride and the strengths of the forces involved. (4)
5. Explain what is meant by the term ‘Heat of vaporisation’. (2)
5.2 Explain why in the early morning just before dawn breaks, the temperature of the
air suddenly drops. (2)
(47)
Gr11_Physical_Science_Term1_Resource_Book.indb 53 2018/12/24 8:24:40 AM
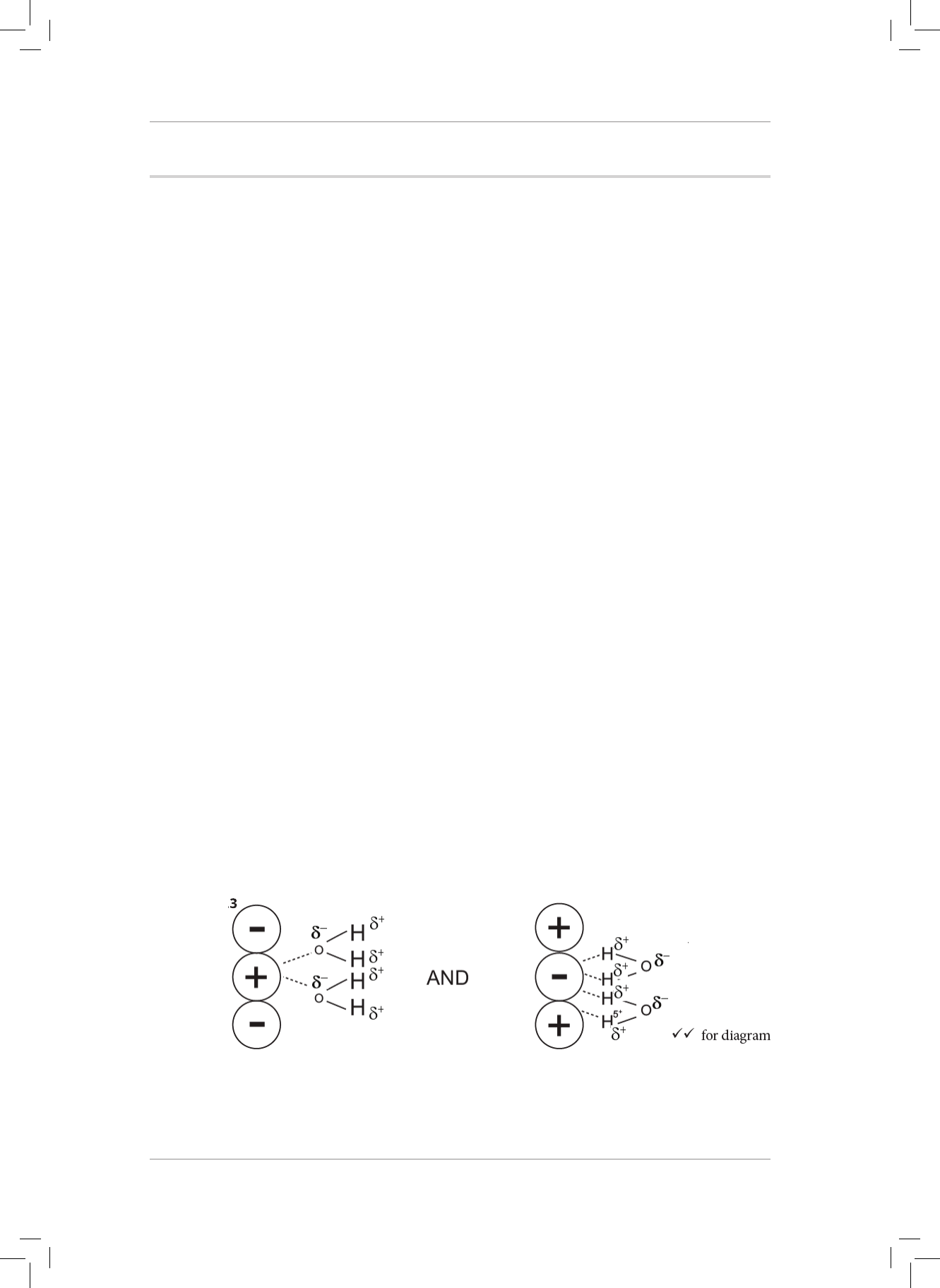
RESOURCE PACK
54
MARKING GUIDELINES
MULTIPLE CHOICE
1. A is question is a direct understanding of the de nition so learners must
learn this. (2)
2. A Sodium chloride is an ionic compound; water is a dipole. (2)
3. B e criteria for a molecule to be able to exert hydrogen bonding
intermolecular forces is when a H atom is bonded to a small atom of high
electronegativity such as N, O or F. (2)
4. D Hexane is a non-polar molecule as it is perfectly symmetrical and thus the
distribution of electrons in the electron cloud is symmetrical hence no region
of δ
+
or δ
-
. (2)
LONG QUESTIONS
1.1 Polar covalent bonding (1)
1.2 e measure of the tendency of an atom to attract a shared pair of electrons
within the chemical bond. (2)
1.3 HCl Electronegativity di erence = 3,5 – 2,1 = 1,4
CaCl
2
Electronegativity di erence = 3.5 – 1,0 = 2,5
Pauling scale states that electronegativity di erence greater than 2,0 will make the
substance ionic in character. CaCl
2
is greater that 2,0 hence will be ionic whereas
HCl at 1,4 will be polar covalent in character as Pauling scale states that from 0 to
2,0, substance will be covalent. (4)
1.4 HCl molecules have two distinct regions of charge δ
+
and δ
-
at either end of the
molecule (2)
1.5 1.5.1 Ionic crystal lattice (1)
1.5.2 a) ion – ion (ionic)
b) hydrogen bonding (dipole)
c) ion – dipole (3)
1.5.3
1.5.3
Polar water molecules surround the ions in the crystal lattice creating an ion-
dipole intermolecular force. A single ion-dipole intermolecular force is not
Gr11_Physical_Science_Term1_Resource_Book.indb 54 2018/12/24 8:24:41 AM

55
WORKSHEETS
strong enough to overcome the very strong ion-ion forces within the lattice.
More water molecules thus move to surround the ions to create a combined ion-
dipole force that will overcome the forces within the crystal lattice. e ions
are then removed from the lattice and go into solution. (5)
2.1 Br
2
or PH
3
Atoms in the molecule with equal electronegativities, hence electro-
negativity dierence of 0, hence a pure covalent bond (non-polar) (1)
2.2 KF is is the only ionic substance in the list and contains cations and
anions. ese are very strong forces of attraction, thus a very high
boiling point (1)
2.3 H
2
O H is bonded to the small O atom which has a high electronegativity. (1)
2.4 SiO
2
is is solid made of covalent bonds only hence called a covalent
network solid. ese covalent bonds are very strong hence a very high
melting point. (1)
2.5 Mg A pure metal with metallic bonding between atoms, Contains
delocalised valence electrons. (1)
2.6 HCl ere is an electronegativity dierence of 1,4 between atoms, hence it is a
dipole.
(1)
3. 1 Non-polar covalent (e electronegativity dierence between two F atoms is 0,
hence will be non-polar). (2)
3.2 All halogen molecules will have London/dispersion intermolecular forces between
them . e size of the halogen molecules increases as one moves down the Table. is
means that the electron cloud density increases allowing for a greater surface area
meaning that there will be more points of contact for intermolecular forces to occur.
ere will be stronger intermolecular forces between the molecules and more energy
needed to overcome these forces. (4)
4.1 KBr and H
2
S (2)
4.2 HCl and CO (2)
4.3 NaCl and CCl
4
(2)
4.4 CCl
4
and Br
2
(2)
5.1 Ice has a lower density compared to liquid water at around temperatures of 4 °C . is
is due to the fact that the hydrogen bonding intermolecular forces are further apart in
the solid phase compared to water in the liquid phase at these very cold temperatures.
(us due to the increase in the distance when the intermolecular forces act in the
solid phase the density of ice decreases and it oats. In the liquid phase at very cold
temperatures, the water molecules can approach each other more closely, hence the
density of water increases at these temperatures). (2)
5.2 Ice will oat, cold water will sink – thus aquatic life can survive at the bottom of the
ocean/river. (1)
Gr11_Physical_Science_Term1_Resource_Book.indb 55 2018/12/24 8:24:41 AM
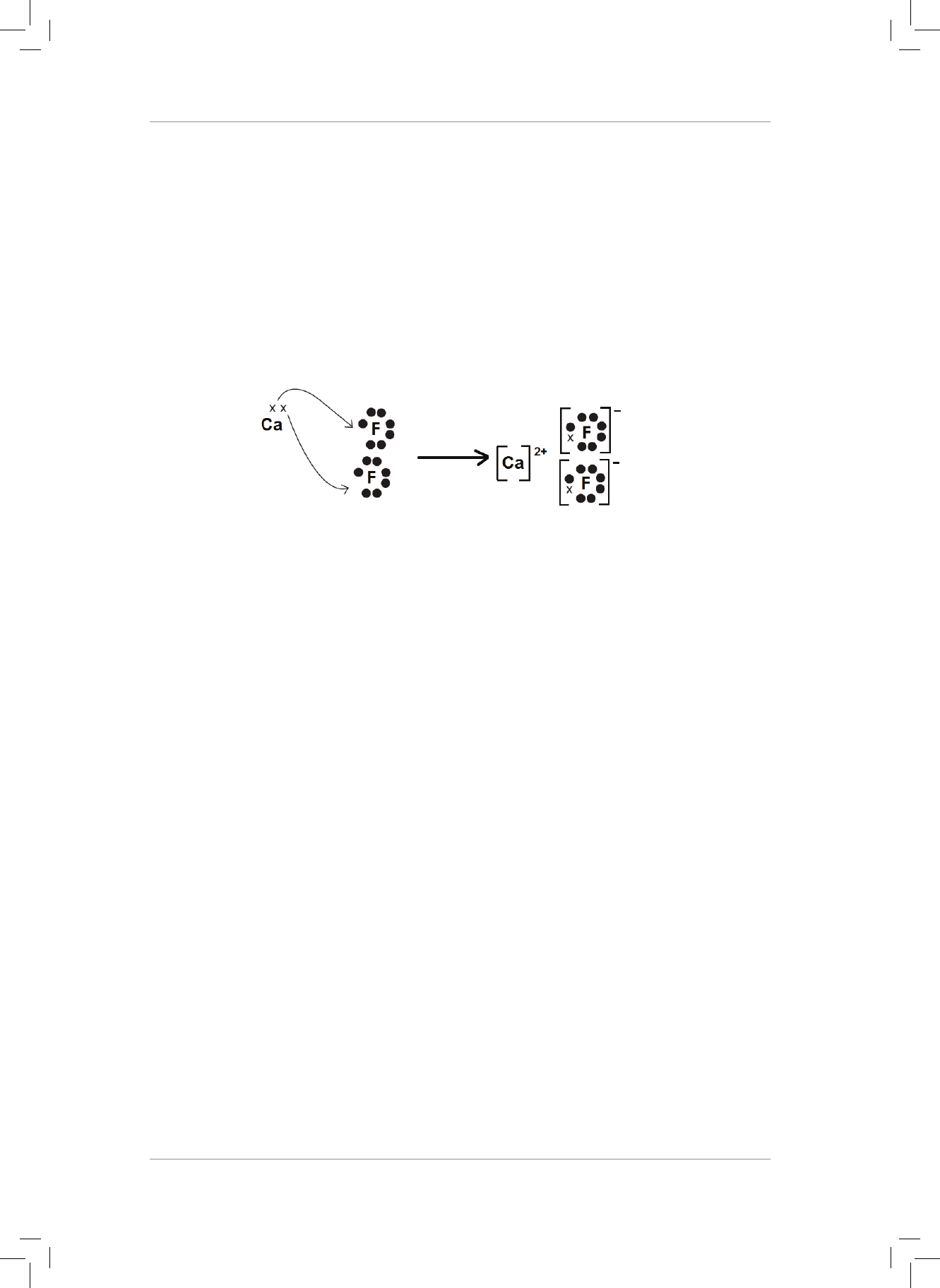
RESOURCE PACK
56
CONSOLIDATION EXERCISE
TOTAL: 47 MARKS
1.1 e measure of the amount of attraction an atom has on a shared pair of electrons
(2)
1.2 HF : electronegativity dierence = 1,9
CaF : electronegativity dierence = 3,0
HF will be expected to be covalent although it does have some ionic character due to
the relatively large electronegativity dierence. CaF
2
will be strongly ionic due to a
very high electronegativity dierence. (4)
1.3
PAGE 54 1.5.3
PAGE 56 QUESTION 1.3
(4)
1.4 δ
+
δ
−
HF has two distinct regions of charge on the molecule H − F (2)
1.5 e H atom in the molecule must be bonded to a small atom of very high
electronegativity. (2)
2.1 Very high melting points indicate that there are very strong intermolecular forces
between the particles in each solid.
(2)
2.2 At 700
o
C, both P and Q are still in the solid phase. e fact that P is able to conduct
electricity tells us it must have charges that are free to move in the solid phase . is
means that P must be a metal with delocalised electrons hence the conductivity . Q
has no free charges in the solid phase thus will not be able to conduct electricity,
thus Q cannot be a metallic substance. Q conducts when molten therefore it is an ionic
substance. (4)
2.3 P = Barium (metallic substance thus has delocalised electrons hence conductivity)
Q = Potassium iodide (ionic substance where the ions are held in the crystal lattice
and not free to move) (2)
3.1 A bond that exists within a molecule between the atoms in that molecule. (1)
3.2 Polar covalent bond (It will be polar due to the electronegativity dierence
between H and O) (2)
3.3 is is the force of attraction between molecules in a system. (2)
3.4 Hydrogen bonding intermolecular force. (1)
3.5 O is a very small atom in terms of its atomic size.
O has a very high electronegativity. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 56 2018/12/24 8:24:41 AM

57
WORKSHEETS
3.6 δ
+
(H will not have any electrons near its nucleus as the high electronegativity of O will
pull the shared electron pair away from the H atom and towards itself, leaving the H
atom δ
+
) (1)
4.1 Force of attraction that occurs between cations and anions in a crystal lattice
(Bond formed by transfer of electrons) (2)
4.2 e Na
+
cations are surrounded by C,
−
anions and vice versa. is means that there are
many strong electrostatic forces of attraction between the cations and anions leading
to very strong inter-particle (inter-ionic) forces. Large amounts of energy will be
needed to overcome these forces, hence a very high melting point. (3)
4.3 a) Ion-dipole intermolecular force (1)
4.3 b) Na
+
– the O end of the water molecule is attracted to the ion. e O end is δ
−
thus it is attracted to a positive ion, hence the sodium cation. (2)
4.3 c) e ion-dipole force between the water molecule and the ion is a strong
intermolecular force, yet is not stronger that the ion-ion electrostatic forces within
the crystal lattice. Water thus needs to accumulate around an ion so that the
combined ion-dipole intermolecular forces eventually will overcome the ion-ion
forces and the ion is only then removed from the lattice and goes into solution. (4)
5.1 is is the amount of energy one mole of water will absorb before it is able to escape to
the gaseous phase
(2)
5.2 Energy is absorbed by the water molecules that sit on grass and plants (dew) just before
the sun rises. is allows the water molecule to escape to the gas phase with a certain
kinetic energy. is means that energy is taken away from the system hence it begins to
feel much colder as there is less energy in the system’s surroundings. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 57 2018/12/24 8:24:41 AM

Gr11_Physical_Science_Term1_Resource_Book.indb 58 2018/12/24 8:24:41 AM
FORMAL
EXPERIMENT
Gr11_Physical_Science_Term1_Resource_Book.indb 59 2018/12/24 8:24:41 AM

FORMAL EXPERIMENT
GRADE 11 TERM 1: PHYSICS
Second Law of Motion
Fa \
60 marks
a
m
1
\
50 marks
Gr11_Physical_Science_Term1_Resource_Book.indb 60 2018/12/24 8:24:43 AM
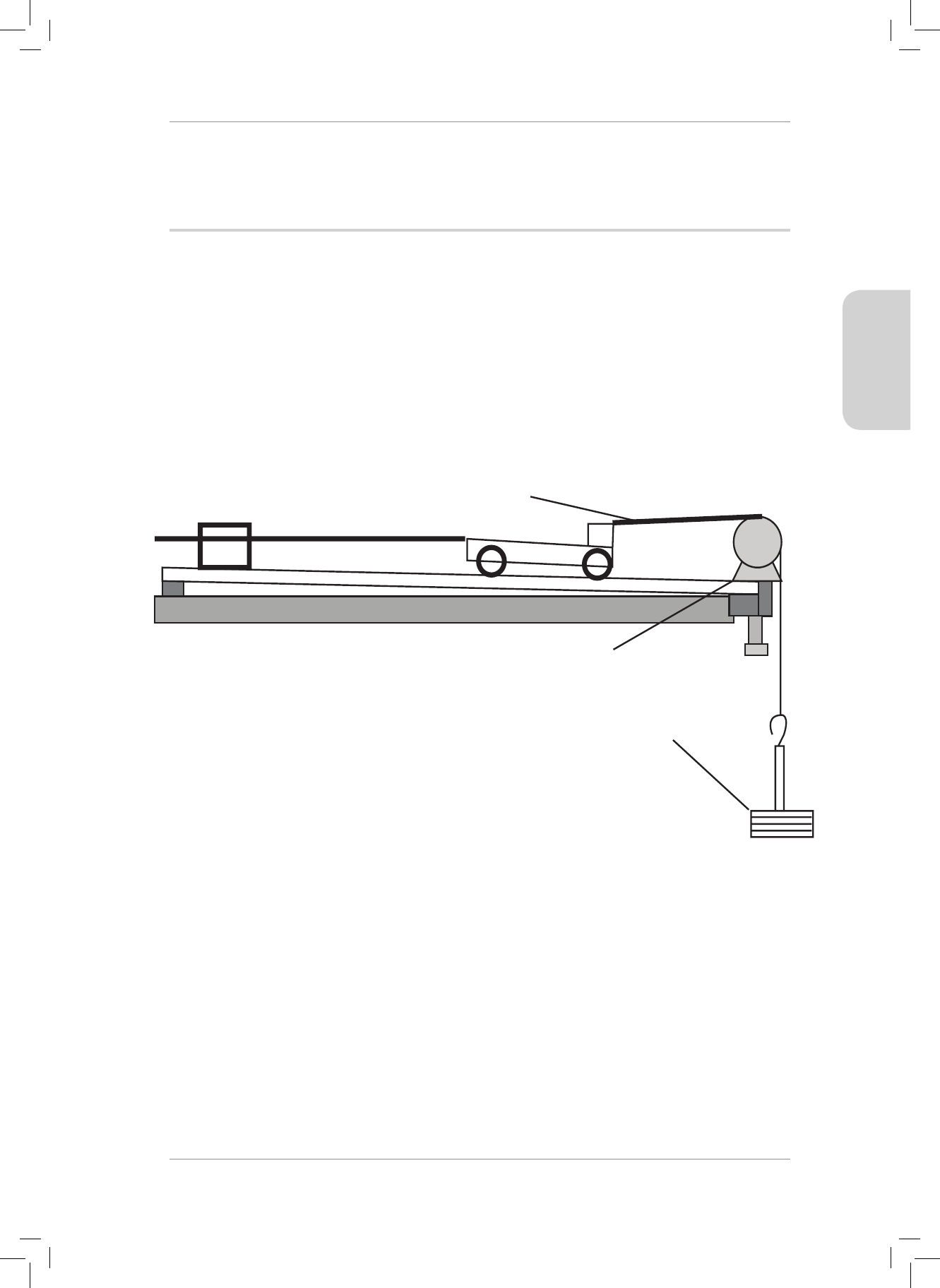
EXPERIMENT
Formal Experiment
TECHNICAL INSTRUCTIONS
Newton’s Second Law of Motion: When a net force, F
net
, is applied to an object of mass, m,
it accelerates in the direction of the net force. e acceleration, a, is directly proportional to
the net force and inversely proportional to the mass.
F
net
= ma
AIM: To verify the relationship between acceleration and
net force of the system of constant mass. 60 marks
APPARATUS:
12 V AC power supply
Conducting leads
Ticker timer
Ticker tape
Trolley track
Trolley
10 g slotted mass holder with 4 × 10 g mass pieces
Light inextensible string
Clamp (to hold the pulley on the track)
Freely running (almost frictionless) pulley
Books (to raise one end of the trolley track)
Mass meter
Prestik
Ticker timer
Ticker tape
Trolley
String
Pulley
Slotted masses
Gr11_Physical_Science_Term1_Resource_Book.indb 61 2018/12/24 8:24:44 AM

RESOURCE PACK
62
METHOD
A. Setting up a friction compensated track
1. Clamp a pulley to the one end of the track.
2. Place a stopper on the track in front of the pulley to stop the trolley before it bumps
into the pulley.
3. Position the track at the edge of a table so that when a weight is hung from the pulley
it can fall freely to the ground.
4. Connect the ticker timer unit to the AC power pack with the two connecting leads.
5. Position the ticker timer unit on the other end of the track. Ensure it is rmly in
place by attaching it to the track with some Prestik.
6. Raise the end of the track which has the ticker timer unit on it by placing a few
books under it.
7. Measure a length of ticker tape equal to the length of the track + 10 cm. Run the
ticker tape through the ticker tape unit and attach the tape to one end of the trolley.
8. Place the trolley on the raised end on the track and hold it in a position which will
allow it to run down the track to the other end.
9. Start the ticker timer and release the trolley, allowing it to run freely down the track
while it pulls the tape through the timer.
10. Stop the timer when the trolley reaches the stopper at the other end.
11. Examine the ticker tape as follows:
11.1 If the gaps between the dots on the tape increase gradually along the length of
the tape, the trolley is accelerating. Lower the height of the track a little (by
removing a book or two). Repeat the procedure from step 6.
11.2 If the gaps between the dots on the tape are of equal length, the trolley is
running at constant velocity. Move on to Part B of the procedure.
B. Investigating the relationship between acceleration and net force on
a system with constant mass.
1. Measure the mass of the trolley.
2. Ensure that the track is stable and secure.
3. Cut a length of string which extends from the trolley at its maximum height and for
about 5 cm over the pulley.
4. Attach one end of the string to the trolley and the other end to the hook of the
slotted mass holder.
Gr11_Physical_Science_Term1_Resource_Book.indb 62 2018/12/24 8:24:44 AM

63
EXPERIMENT
5. Place four of the slotted mass pieces on the trolley.
6. Feed a new length of ticker tape through the ticker timer and attach it to the trolley.
Label the tape with the mass of the slotted mass holder (and the mass pieces on it).
7. Hold the trolley on the track while you position the string over the pulley. Hook the
slotted mass holder onto the other end of the string and allow it to hang freely over
the edge of the table.
8. Start the timer and release the trolley and the mass holder so that the trolley
accelerates down the track as the mass holder falls to the ground.
9. Repeat steps 6 to 8 four more times, by moving one of the four slotted mass pieces
from the trolley to the mass holder each time.
Gr11_Physical_Science_Term1_Resource_Book.indb 63 2018/12/24 8:24:44 AM
RESOURCE PACK
64
NAME: GRADE:
Formal Experiment
NEWTON’S SECOND LAW OF MOTION
THEORY
Newton’s Second Law of Motion: When a net force, F
net
, is applied to an object of mass, m,
it accelerates in the direction of the net force. e acceleration, a, is directly proportional to
the net force and inversely proportional to the mass.
F
net
= ma
AIM: To verify the relationship between acceleration and
net force of the system of constant mass. 60 marks
APPARATUS
12 V AC power supply
Conducting leads
Ticker timer
Ticker tape
Trolley track
Trolley
10 g slotted mass holder with 4 × 10 g mass pieces
Light inextensible string
Clamp (to hold the pulley on the track)
Freely running (almost frictionless) pulley
Books (to raise one end of the trolley track)
Mass meter
Prestik
Gr11_Physical_Science_Term1_Resource_Book.indb 64 2018/12/24 8:24:44 AM
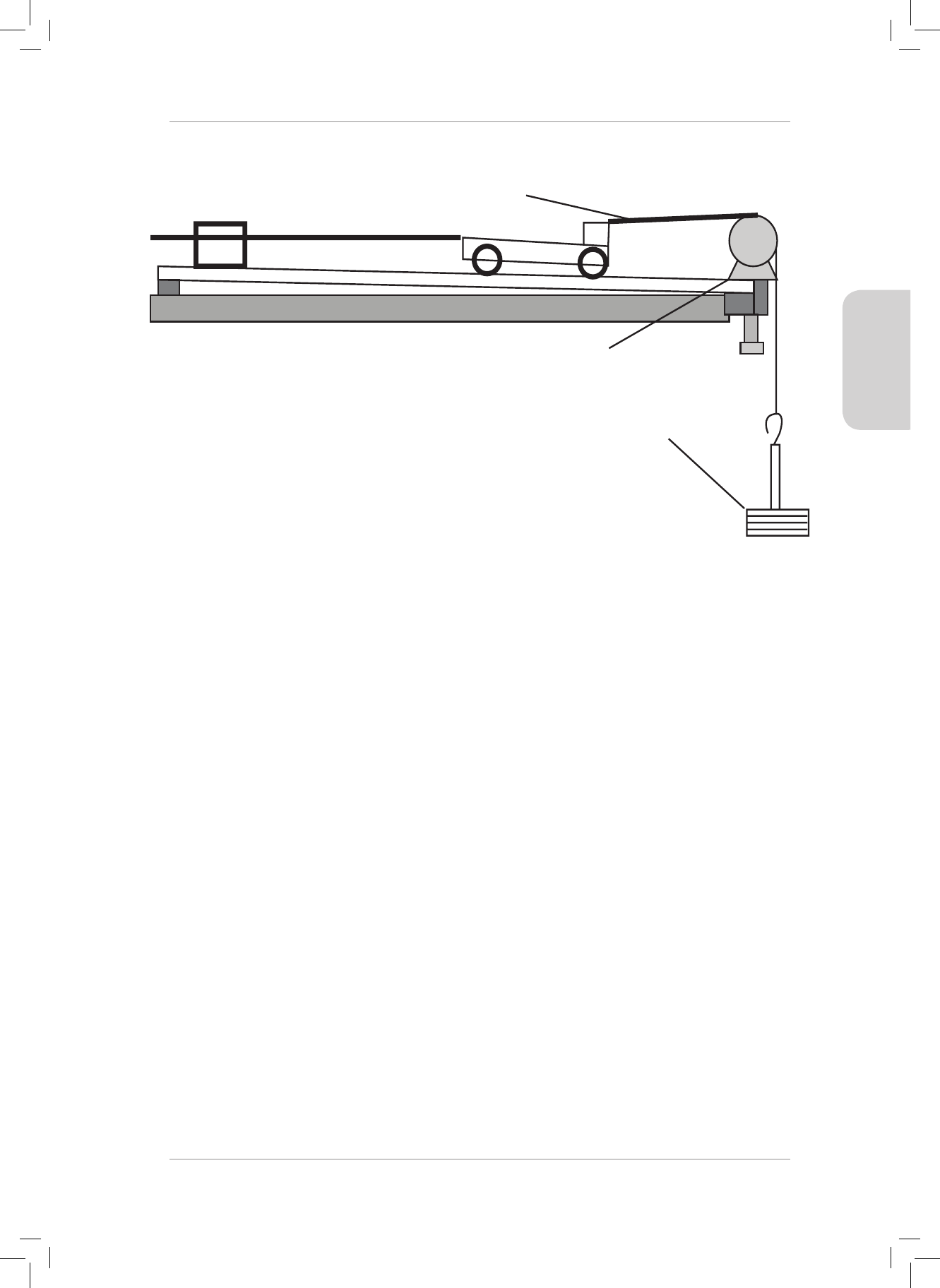
65
Ticker timer
Ticker tape
Trolley
String
Pulley
Slotted masses
EXPERIMENT
METHOD
1. Clamp a pulley to the one end of the track.
2. Place a stopper on the track in front of the pulley to stop the trolley before it bumps
into the pulley.
3. Position the track at the edge of a table so that when a weight is hung from the pulley
it can fall freely to the ground.
4. Connect the ticker timer to the AC power pack with the two connecting leads.
5. Position the ticker timer on the other end of the track. Ensure it is rmly in place by
attaching it to the track with some Prestik.
6. Raise the end of the track which has the ticker timer unt on it by placing a few books
under it.
7. Measure a length of ticker tape equal to the length of the track + 10 cm. Run the
ticker tape through the ticker timer and attach the tape to one end of the trolley.
8. Place the trolley on the raised end on the track and hold it in a position which will
allow it to run down the track to the other end.
9. Start the ticker timer and release the trolley, allowing it to run freely down the track
while it pulls the tape through the timer.
10. Stop the timer when the trolley reaches the stopper at the other end.
11. Examine the ticker tape as follows:
Gr11_Physical_Science_Term1_Resource_Book.indb 65 2018/12/24 8:24:45 AM
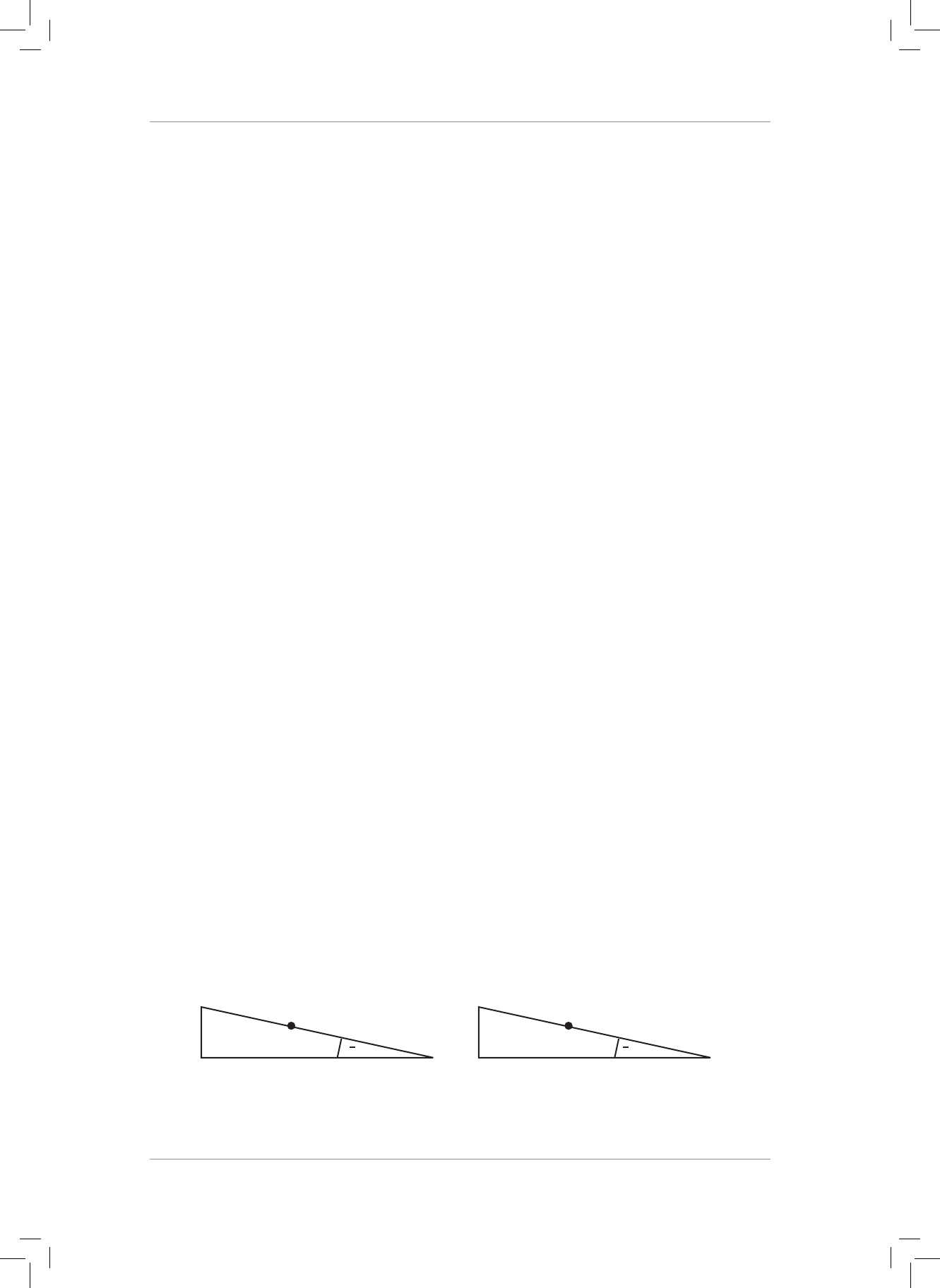
RESOURCE PACK
66
11.1 If the gaps between the dots on the tape increase gradually along the length of
the tape, the trolley is accelerating. Lower the height of the track a little (by
removing a book or two). Repeat the procedure from step 6.
11.2 If the gaps between the dots on the tape are of equal length, the trolley is
running at constant velocity. Move on to Part B of the procedure.
a system with constant mass.
1. Measure the mass of the trolley.
2. Ensure that the track is stable and secure.
3. Cut a length of string which extends from the trolley at its maximum height and for
about 5 cm over the pulley.
4. Attach one end of the string to the trolley and the other end to the hook of the
slotted mass holder.
5. Place four of the slotted mass pieces on the trolley.
6. Feed a new length of ticker tape through the ticker timer and attach it to the trolley.
Label the tape with the mass of the slotted mass holder (and the mass pieces on it).
7. Hold the trolley on the track while you position the string over the pulley. Hook the
slotted mass holder onto the other end of the string and allow it to hang freely over
the edge of the table.
8. Start the timer and release the trolley and the mass holder so that the trolley
accelerates down the track as the mass holder falls to the ground.
9. Repeat steps 6 to 8 four more times, by moving one of the four slotted mass pieces
from the trolley to the mass holder each time.
OBSERVATIONS AND RESULTS
1. Draw and label the forces acting on the trolley when it is a) running at constant
velocity on the track and b) accelerating down the track.
a. At constant velocity on the track b. Accelerating on the track
0 0
(7)
Gr11_Physical_Science_Term1_Resource_Book.indb 66 2018/12/24 8:24:45 AM
67
EXPERIMENT
2. Name the independent variable: ___________________________________ (1)
3. Name the dependent variable: ___________________________________ (1)
4. Name two control variables: ___________________________________ (2)
5. Analyse each ticker tape as follows:
5.1 Choose a section of tape where the gaps between the dots are easy to see.
5.2 Mark o consecutive segments of ve gaps between dots until you have three
consecutive segments of ve gaps on each tape.
5.3 Record the length of each of the segments for each tape in the table below.
5.4 Calculate the average speed in the rst and last segment.
5.5 Calculate the acceleration of the trolley.
1
2
3
1
()v
1
,
Segment
01
1
3
()v
3
,
Segment
01
3
,
vv
02
31
-
(17)
ANALYSIS AND INTERPRETATION
6. Plot a graph of acceleration against force using the graph paper provided. (7)
Gr11_Physical_Science_Term1_Resource_Book.indb 67 2018/12/24 8:24:46 AM
RESOURCE PACK
68
7. Extend the graph so that it cuts the y-axis.
Gr11_Physical_Science_Term1_Resource_Book.indb 68 2018/12/24 8:24:47 AM

69
EXPERIMENT
8. Comment on the shape of the graph. (2)
Deduce the relationship between the acceleration of the trolley and the net force
acting on the trolley. (5)
9. Calculate the gradient of the graph. Show the coordinates that you use for this
calculation on the graph. (4)
10. What quantity does the gradient of the graph represent? Explain briey. (4)
11. Compare the value of the gradient of the graph with the total mass of the
accelerating system. Calculate the % error. (Total mass = 300 g) (6)
CONCLUSION
12. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 69 2018/12/24 8:24:47 AM

RESOURCE PACK
70
13. Comment on the experimental procedure:
Give a suggestion of how to take more reliable results. (1)
PART C 50 MARKS
AIM: To verify the relationship between acceleration and mass of the
system when a constant net force acts on it.
VARIABLES
Independent variable: _________________________________________________ (2)
Dependent variable: __________________________________________________ (2)
Control variable: _____________________________________________________ (2)
METHOD
Write down the method for this part of the investigation. (5)
Gr11_Physical_Science_Term1_Resource_Book.indb 70 2018/12/24 8:24:47 AM
EXPERIMENT
OBSERVATIONS AND RESULTS
1. Table of results: Acceleration of the system vs mass for a constant net force
Calculate and complete the values missing from the table of results. (20)
m
m
1
bl
−1
1
2
3
1
()v
1
,
Segment
01
1
3
()v
3
,
Segment
01
3
,
vv
02
31
-
0,300 0,012 0,029 0,045
0,290 0,031 0,048 0,065
0,280 0,045 0,063 0,080
0,270 0,021 0,039 0,057
0,260 0,056 0,075 0,094
ANALYSIS AND INTERPRETATION
4. Plot a graph of acceleration against the inverse of mass (on the graph paper which is
available on the next page). (7)
5. Describe the shape of the graph. (2)
6. Describe the relationship between acceleration and the inverse of mass. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 71 2018/12/24 8:24:48 AM
RESOURCE PACK
72
Gr11_Physical_Science_Term1_Resource_Book.indb 72 2018/12/24 8:24:48 AM

73
EXPERIMENT
6. Give a reason why it is much more useful to plot a graph of acceleration against the
inverse of mass, than it is to plot a graph of acceleration against mass. (5)
CONCLUSION
7. (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 73 2018/12/24 8:24:48 AM
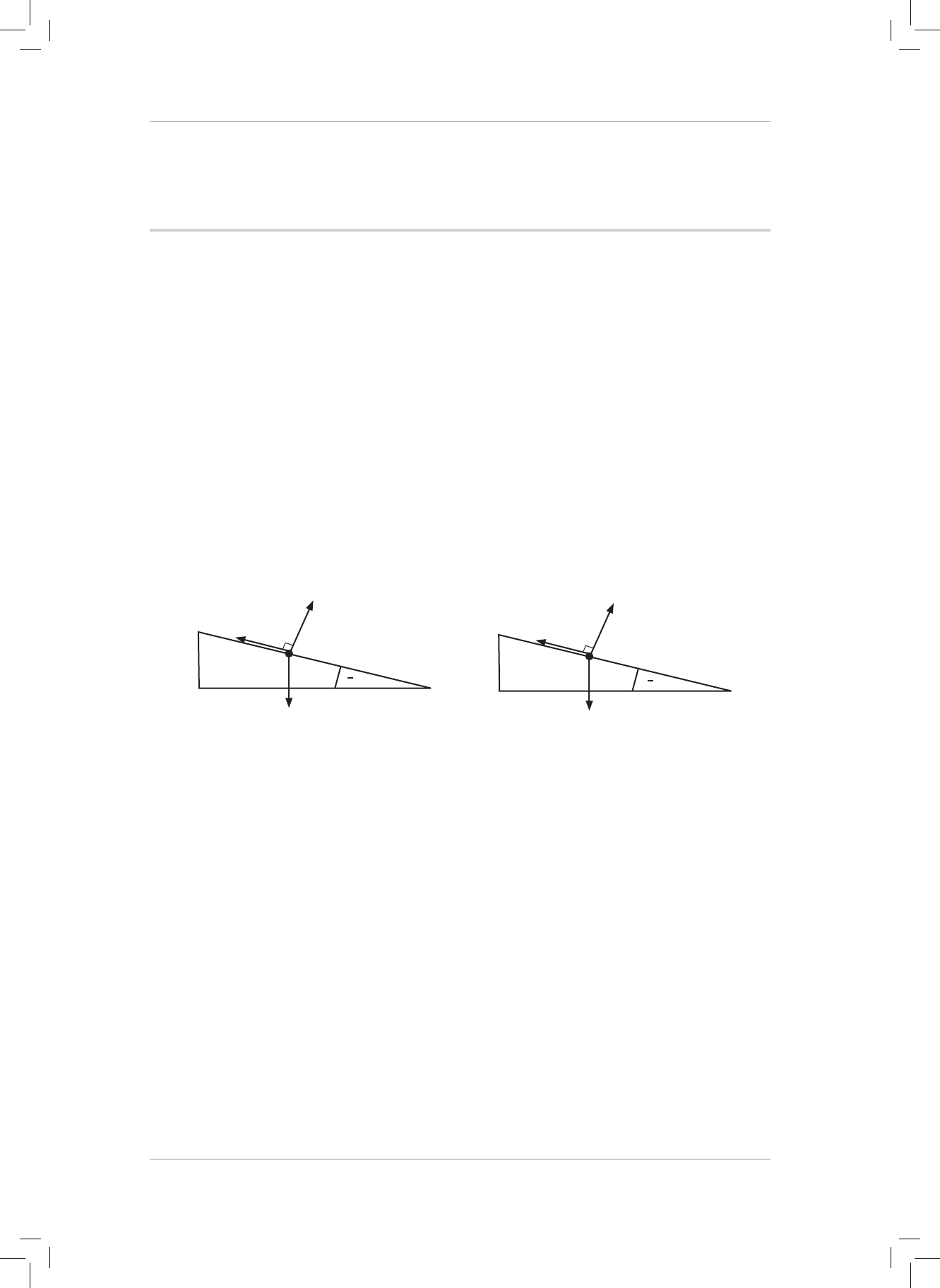
RESOURCE PACK
74
Formal Experiment
MARKING GUIDELINES
110 MARKS
Newton’s Second Law of Motion: When a net force, F
net, is applied to an object of mass, m,
it accelerates in the direction of the net force. e acceleration, a, is directly proportional to
the net force and inversely proportional to the mass.
F
net = ma
PART B 50 MARKS
AIM: To verify the relationship between acceleration and
net force on a system of constant mass.
OBSERVATIONS AND RESULTS
1. Draw and label the forces acting on the trolley when it is a) running at constant velocity
on the track and b) accelerating down the track.
a. At constant velocity on the track b. Accelerating on the track
0
0
Normal force
Normal force
Friction
Friction
Weight
Weight
No net force Net force > 0 (7)
2. Name the independent variable: Net force (1)
3. Name the dependent variable: Acceleration (1)
4. Name two control variables: Mass of the system (2)
Angle of the inclined plane
(friction compensated plane)
5. Analyse each ticker tape as follows:
5.1 Choose a section of tape where the gaps between the dots are easy to see.
5.2 Mark o consecutive segments of ve gaps between dots until you have three
consecutive segments of ve gaps on each tape.
5.3 Record the length of each of the segments for each tape in the table below.
5.4 Calculate the average speed in the rst and last segment.
5.5 Calculate the acceleration of the trolley. (18)
Gr11_Physical_Science_Term1_Resource_Book.indb 74 2018/12/24 8:24:49 AM
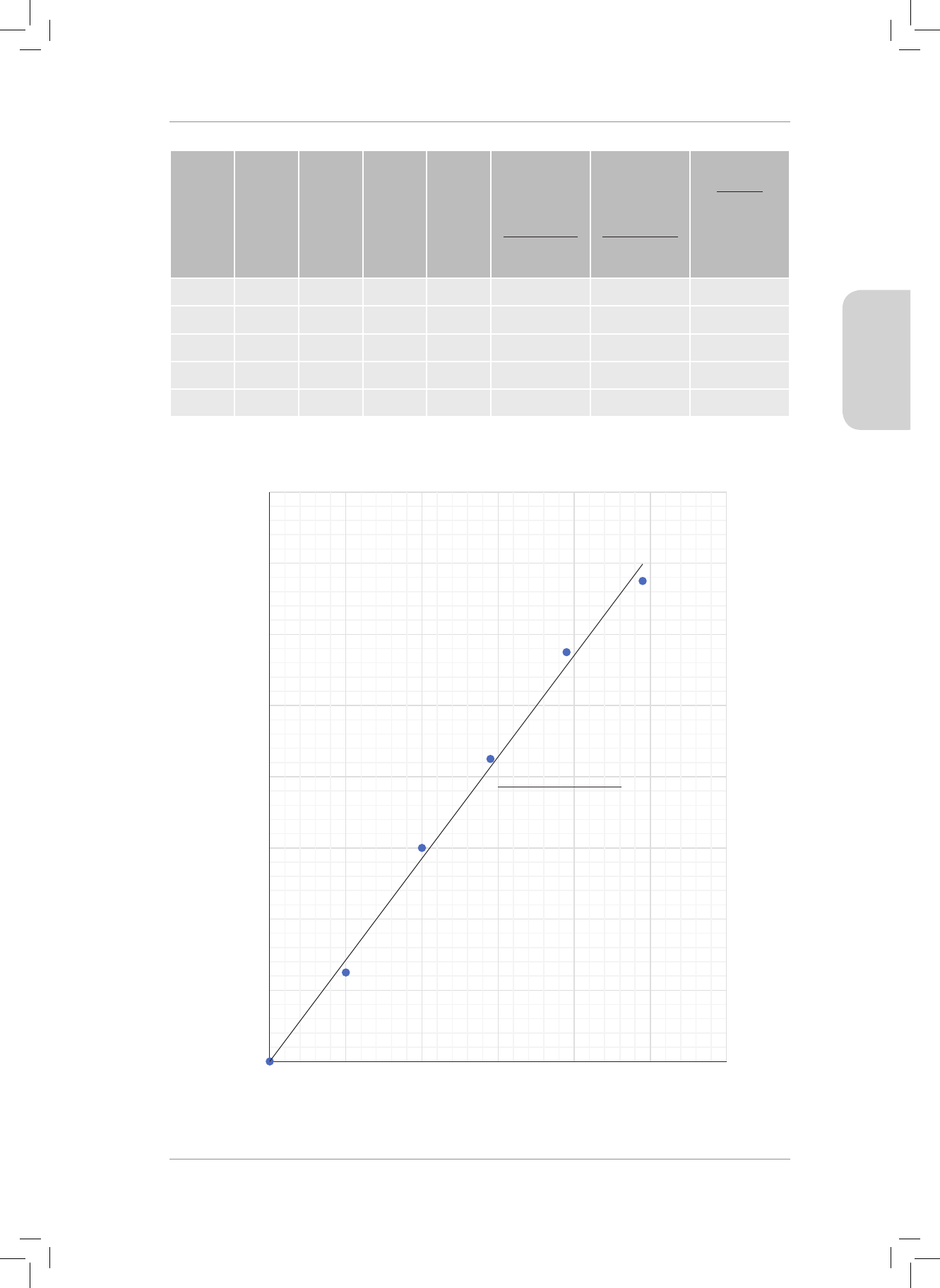
75
EXPERIMENT
1
2
3
()v
1
,
Segment
01
1
()v
3
,
Segment
01
3
,
vv
02
31
-
0,010
0,10
39,0 41,0 44,0
0,39
0,44 0,25
0,20 0,20 54,0 61,0 66,0 0,54 0,66
0,60
0,030
0,29
62,0 68,0 79,0
0,62
0,79 0,85
0,040 0,39 86,0 98,0 109,0 0,86 1,09
1,15
0,050 0,49 90,5 105,0 118,0 0.91 1,18
1,35
y = 2,8529x
0,00
0,20
0,40
0,60
0,80
1,00
1,20
1,40
1,60
0,00 0,10 0,20 0,30 0,40 0,50 0,60
Acceleration (m⋅s
-2
)
Net force (N)
Acceleration vs Net force
for a trolley system of fixed mass
Marking the graph: (7)
Appropriate title (heading) e.g.
Acceleration (of the system) against net
force (for constant mass).
Correct choice of axes: Independent
variable on x-axis (c.o.e.)(Net force)
Appropriate scale on x-axis with label and
SI units
Appropriate scale on y-axis with label and
SI units
Points plotted correctly.
Best t straight-line graph.
Extended back to cut the y-axis (Must be
the same straight-line as the graph)
Gr11_Physical_Science_Term1_Resource_Book.indb 75 2018/12/24 8:24:49 AM

RESOURCE PACK
76
8. Comment on the shape of the graph.
A straight-line graph cuts the y-axis at the origin. (2)
Deduce the relationship between the acceleration of the trolley and the net force acting
on the trolley.
e equation of the graph is y = mx where m is the gradient of the graph
Y-values represent acceleration of the trolley.
X-values represent the net force.
Since the graph passes through the origin, the acceleration is directly proportional to the
net force acting on it.
(5)
9. Calculate the gradient of the graph. Show the coordinates that you use on the graph.
or
x
y
D
D
method
Using the appropriate values of the two coordinates
shown on the graph
,,
,,
,
gradient
F
a
04900
14000
286!
D
D
=
=
-
-
=
accurate calculation SI units not required. (4)
10. What quantity does the gradient of the graph represent? Explain briey.
Fma
=
therefore
a
m
F
=
(Newton’s second law)
From the graph:
akF
=
where k is the gradient of the graph.
erefore
k
m
1
=
OR e gradient equals the inverse of the mass of the system. (4)
11. Compare the value of the gradient of the graph with the total mass of the accelerating
system. Calculate the % error.
Find the inverse of the gradient (0,350)
Compare inverse of gradient with the total mass of the accelerating system.
Comment on % error
% error = × 100%
method
= × 100% (for example)
= 16,7 %
Fairly low percentage error (therefore results are reasonably reliable.) (6)
Total mass of system - inverse of gradient
Total mass of system
0,300 - 0,350
0,300
Gr11_Physical_Science_Term1_Resource_Book.indb 76 2018/12/24 8:24:50 AM

77
EXPERIMENT
CONCLUSION
12. e acceleration is directly proportional to the net force provided the mass of the system
remains constant.
(2)
13. Comment on the experimental procedure:
ANY VALID SUGGESTION e.g.
e results would be more reliable if for each set of readings three ticker tapes were run
through.
e average of the acceleration could be calculated. is would give a more reliable result.
(1)
PART C 50 MARKS
AIM: To verify the relationship between acceleration and mass of the
system when a constant net force acts on it.
1. VARIABLES
Independent variable: Mass (of the system)
(2)
Dependent variable: Acceleration (of the system)
(2)
Control variable: Constant net force
(2)
2. METHOD
Write down the method for this part of the investigation.
Learners will use their own words – they must essentially plan in a similar way. (5)
1. Hang one slotted mass on the mass hanger.
2. Place the remainder of the slotted mass pieces on the trolley.
3. Attach the ticker tape and the mass hanger to the trolley.
4. Allow the trolley to accelerate down the track.
5. Repeat steps 3 and 4 four more times, removing one mass piece from the trolley each time,
until the trolley runs down the track without any mass pieces on it.
Gr11_Physical_Science_Term1_Resource_Book.indb 77 2018/12/24 8:24:50 AM
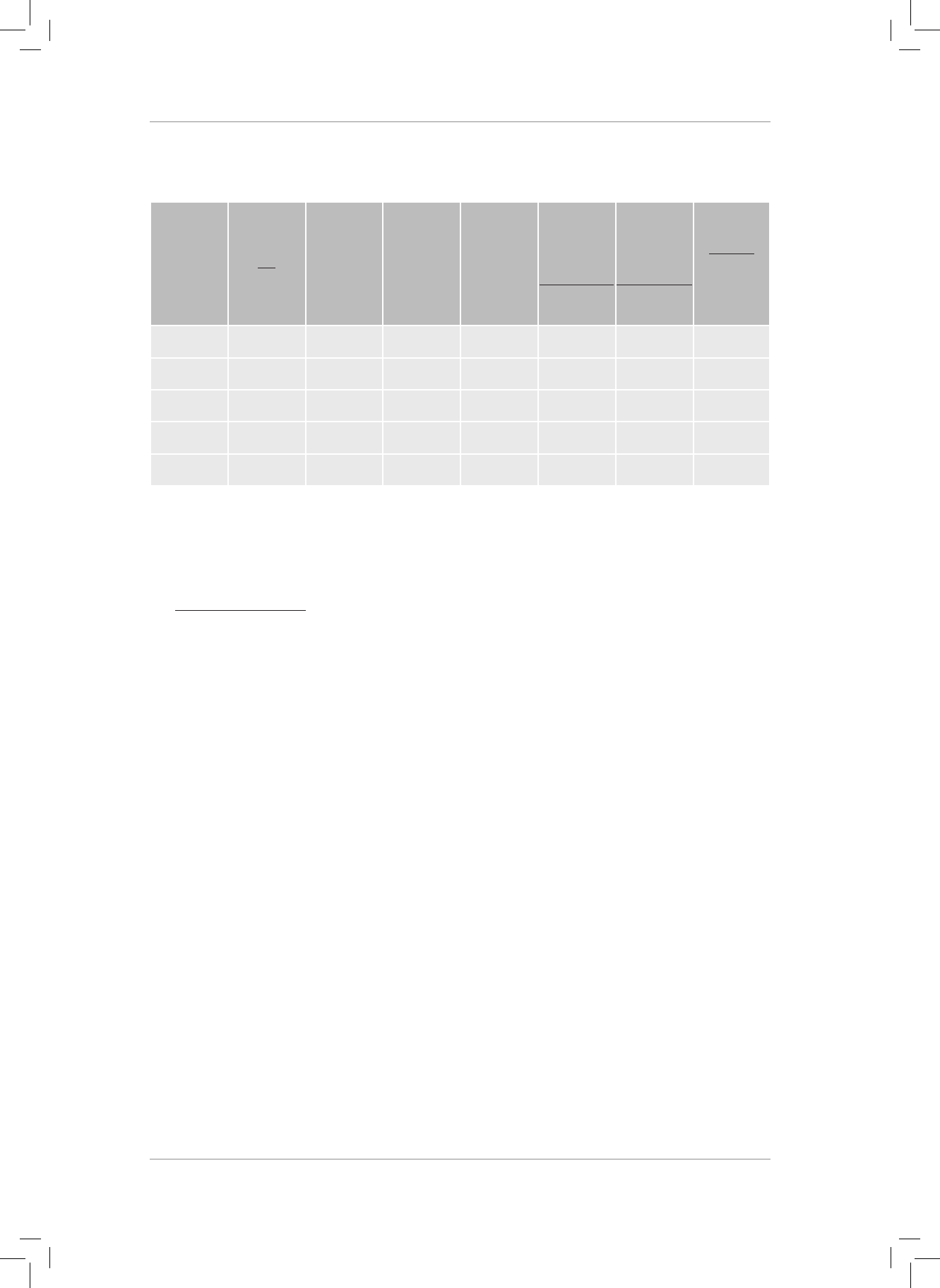
RESOURCE PACK
78
OBSERVATIONS AND RESULTS
3. (20)
m
1
bl
−1
1
()v
1
,
Segment
01
1
3
()v
3
,
Segment
01
3
,
vv
02
31
-
0,300 3,33
0,012 0,029 0,045 0,12 0,45 1,64
0,290 3,45 0,031 0,048 0,065 0,31 0,65 1,70
0,280 3,57 0,045 0,063 0,080 0,45 0,80 1,75
0,270 3,70 0,021 0,039 0,057 0,21 0,57 1,80
0,260 3,85 0,056 0,075 0,094 0,56 0,94 1,90
ANALYSIS AND INTERPRETATION
4. Plot a graph of acceleration against the inverse of mass (on the graph paper which is
available on the next page.
Marking the graph: (7)
Appropriate title (heading) e.g. Graph of acceleration (of the system) against net force
(for constant mass).
Correct choice of axes: Independent variable on x-axis (c.o.e.)
Appropriate scale on x-axis with label and SI units
Appropriate scale on y-axis with label and SI units
Points plotted correctly
Best t straight-line graph
Extended back to cut the y-axis (Must be the same straight-line as the graph)
Gr11_Physical_Science_Term1_Resource_Book.indb 78 2018/12/24 8:24:50 AM
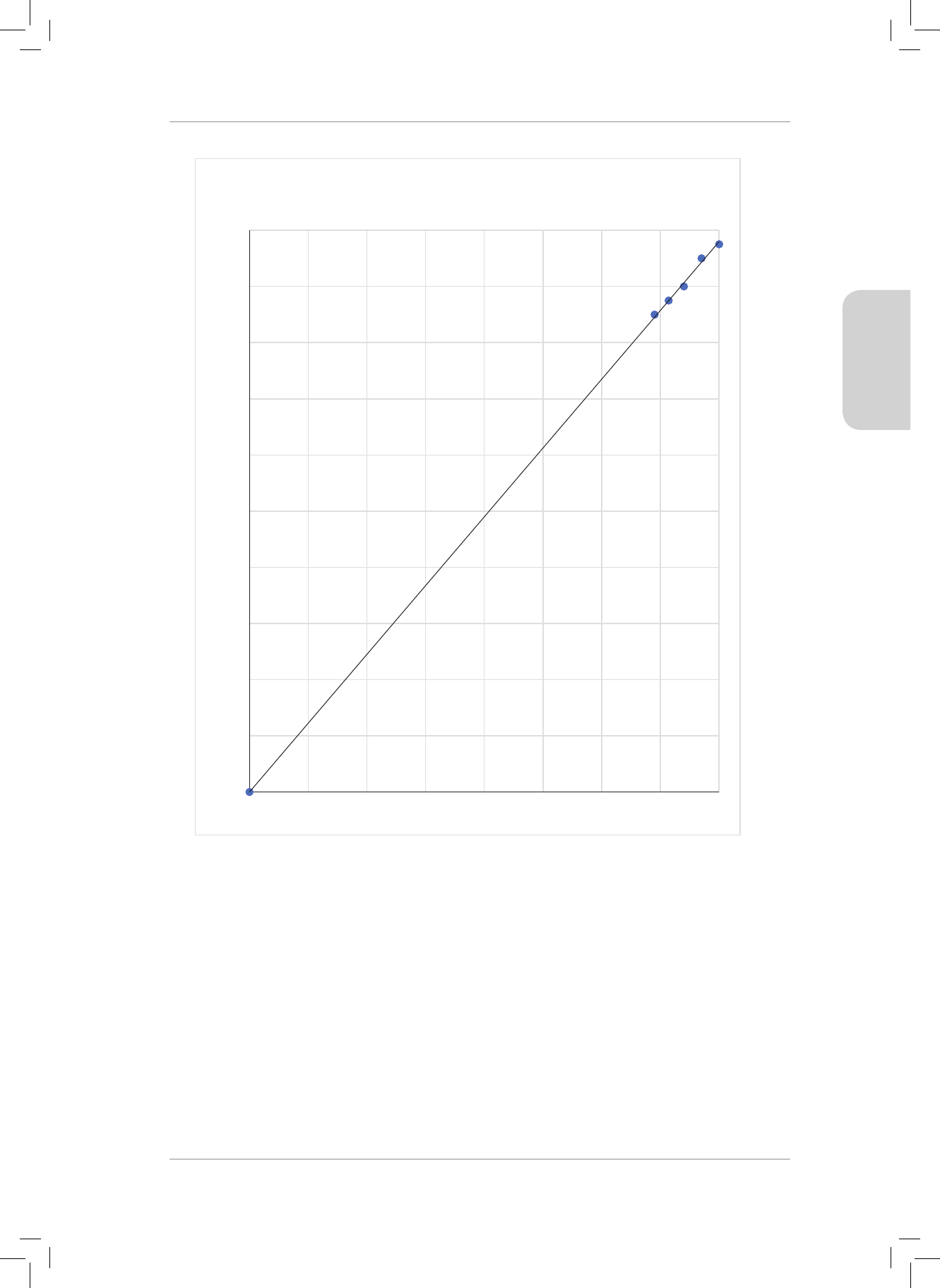
79
EXPERIMENT
PAGE 82 QUESTION 4
0,00
0,20
0,40
0,60
0,80
1,00
1,20
1,40
1,60
1,80
2,00
0,00 0,50 1,00 1,50 2,00 2,50 3,00 3,50 4,00
Acceleration (m⋅s
-2
)
Inverse of mass (kg
-1
)
Acceleration vs Inverse of mass
5. Describe the shape of the graph.
e graph is a straight-line graph
passing through the origin. (2)
6. Describe the relationship between acceleration and the inverse of mass.
e acceleration of the system is directly proportional
to the inverse of its mass
(provided the force remains constant). Remains constant.)
OR e acceleration of the system is inversely
proportional to its mass
(provided the force remains constant). (2)
Give a reason why it is much more useful to plot a graph of acceleration against the
inverse of mass, than to plot a graph of acceleration against mass.
e straight-line graph through the origin conrms that y = mx therefore we know that
acceleration and mass are inversely proportional to each other.
If we had plotted acceleration against mass the graph would have been a hyperbola
Gr11_Physical_Science_Term1_Resource_Book.indb 79 2018/12/24 8:24:51 AM

RESOURCE PACK
80
However to conrm the inverse relationship we should then plot the graph of acceleration
against the inverse of mass.
By plotting it in the rst place we have saved time and eort.
(5)
7. CONCLUSION:
e acceleration of the system is inversely
proportional to its mass
provided the net force remains constant. (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 80 2018/12/24 8:24:51 AM
ASSESSMENTS
Gr11_Physical_Science_Term1_Resource_Book.indb 81 2018/12/24 8:24:51 AM

RESOURCE PACK
82
Topic 1: Vectors in two dimensions
QUESTIONS
MULTIPLE CHOICE
1. e following three forces act on an object in the horizontal plane. A 30 N force
acts to the right, a 50 N force acts to the le and a 40 N force acts to the right. e
resultant force acting on the object is ...
A 120 N.
B 20 N.
C 20 N to the right.
D 60 N to the le. (2)
2. Two forces of magnitude 50 N and 80 N act on an object. Which of the following
forces cannot be the magnitude of the resultant of these two forces?
A 30 N
B 50 N
C 130 N
D 20 N (2)
3. e following two forces act on an object: A 180 N horizontal force acts to the
right and a 100 N force acts upwards in the vertical plane. e magnitude of the
resultant force acting on the object is ...
A 280 N.
B 80 N.
C 205,91 N.
D 0 N. (2)
4. A man hikes 8 km east and 6 km north. e direction of his resultant displacement is:
A on a bearing of 036,9°.
B on a bearing of 053,1°.
C 53,1° north of east.
D 36,9° east of north. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 82 2018/12/24 8:24:51 AM
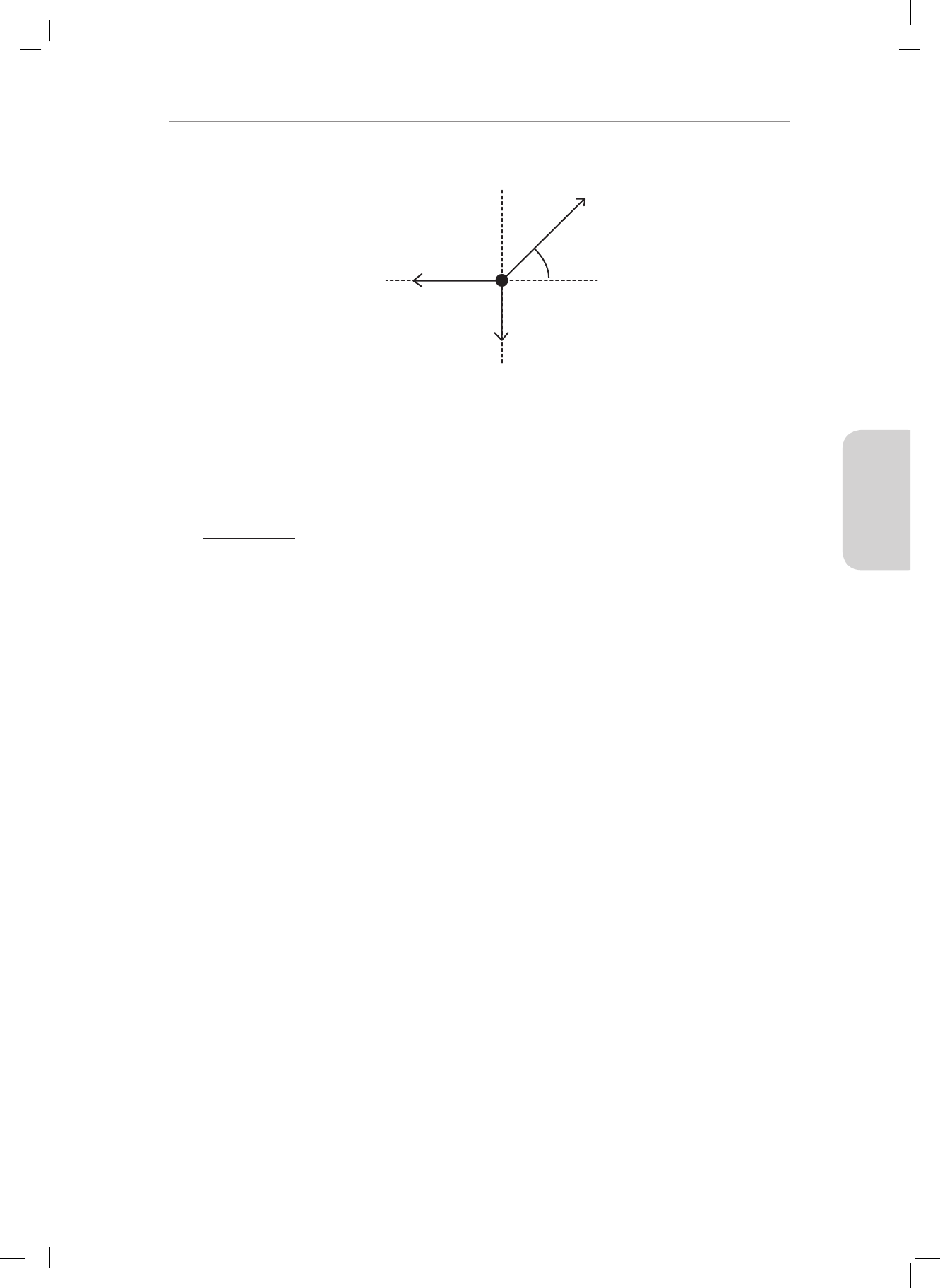
83
ASSESSMENTS
5. ree forces F, T and W act on an object as shown in the diagram below.
T = 200 N
W = 180 N
F = 350 N
60˚
e magnitude and direction of the resultant force in the horizontal plane is ...
A 150 N to the right.
B 200 N to the le.
C 175 N to the right.
D 25 N to the le. (2)
6. Refer to question 1.5. e magnitude and direction of the resultant force in the
vertical plane is ...
A 180 N down.
B 123,11 N upwards.
C 170 N upwards.
D 123,11 N downwards. (2)
LONG QUESTIONS
1. A horizontal force of 300 N is applied to an object to the right while an upward
vertical force of 400 N is applied at the same time.
1.1 Dene a vector. (2)
1.2 Dene a resultant vector. (2)
1.3 Draw a neat, fully labelled vector diagram of these forces in the Cartesian
plane, using the tail-to-head method. (2)
1.4 Determine the magnitude and direction of the resultant force acting on the
object. (4)
[10]
2. e following four forces act on an object. A 40 N force acts vertically upwards,
a 30 N force acts horizontally to the right, a 90 N force acts at 45° below the
horizontal and to the le and a 50 N force acts horizontally to the le.
2.1 Draw a neat, fully labelled vector diagram of the four forces in the Cartesian
plane (not necessarily to scale). (4)
2.2 Determine the magnitude and direction of the horizontal and vertical
components of the 90 N force. (6)
2.3 Determine the magnitude and direction of the resultant force acting on the
object. (5)
[15]
Gr11_Physical_Science_Term1_Resource_Book.indb 83 2018/12/24 8:24:53 AM

RESOURCE PACK
84
3. An aircra ies 30 km north, then 40 km east, then 60 km south and then 60 km west.
3.1 Dene displacement. (2)
3.2 Draw a neat, fully labelled vector diagram (tail-to-head method) of these
displacements. Draw in the resultant displacement (R). (5)
3.3 Draw a neat, fully labelled vector diagram (tail-to-tail method) of
the resultant horizontal (R
x
) and vertical (R
y
) components of these
displacements. Draw in the resultant displacement (R). (5)
3.4 Calculate the magnitude and direction of the resultant displacement of the
aircra. State the direction as a bearing. (4)
[16]
4. A large crate is pulled along horizontal ground by a chain which makes an angle
of 30° above the ground. e chain applies a force of 500 N.
4.1 Draw a neat, fully labelled vector diagram of the force applied by the chain
as well as the horizontal and vertical components of the 500 N force. (3)
4.2 Calculate the magnitude of the force with which the crate is pulled along the
ground by the chain. (2)
4.3 Calculate the magnitude of the force with which the crate is lied by means
of the chain. (2)
4.4 List any other forces which act on the crate in the horizontal and vertical
planes. (3)
[10]
5. ree forces act on an object. F
1
= 35 N acts vertically upwards, F
2
= 65 N acts at
45° above the horizontal and to the right, F
3
= 50 N acts 30° below the horizontal
and to the right.
5.1 Distinguish between a vector and a scalar quantity. (3)
5.2 Draw a neat, fully labelled vector diagram of the three forces in the Cartesian
plane (acting at the origin). (3)
5.3 Determine the magnitude and direction of the resultant force acting on the
object. Draw any relevant vector diagrams to support your answer. (9)
[15]
6. Consider the following three forces which act simultaneously on the same object:
F
1
= 600 N acts on a bearing of 330°, F
2
= 400 N acts on a bearing of 270°,
F
3
= 400 N acts on a bearing of 190°.
6.1 Use a ruler, pencil, protractor and a scale of 1 cm : 100 N to nd the
magnitude and direction of the resultant force (using the tail-to-head
method). Label your vector diagram clearly. (10)
6.2 State the magnitude and direction of a fourth force F
4
which could be
applied to the object so that the resultant force on the object becomes zero. (2)
[12]
Gr11_Physical_Science_Term1_Resource_Book.indb 84 2018/12/24 8:24:53 AM
85
ASSESSMENTS
MARKING GUIDELINES
MULTIPLE CHOICE
1. C
() NR 30 50 40 20
=+ +- ++ =+
^^hh
to the right [CL2] (2)
2. D e maximum resultant force is achieved when the two forces act in the
same direction:
NR 50 80 130
=+ ++ =+
^^hh
e minimum resultant force is achieved when the two forces act in
opposite directions:
NR 80 50 30
=+ +- =+
^^hh
Any resultant force between 30 N and 130 N can be achieved by
increasing the angle between the two forces, from 0° to 180°.
It is impossible to obtain a resultant of 20 N from these two forces. [CL2] (2)
3. C
180 N
180 N
R
100 N100 N
R 100 180
222
=+
R 42 400
2
=
,NR 42 400 205 91
==
[CL2] (2)
4. B
8 km
6 km
ϴ
x
,tan
8
6
36 9
1
ci
==
-
ak
Bearing is measured clockwise from north, therefore the bearing is
90° – 36,9° = 053,1° [CL3] (2)
5. D
coscos NFF 350 60 175
x
ci
== =
to the right
NR 175 200 25
x
=+ -=-
therefore
NR 25
x
=
to the le [CL2] (2)
6. B
,sinsin NFF 350 60 303 11°
y
i
== =
upwards
,,NR 303 11 180 123 11
y
=+ -=+
upwards [CL2] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 85 2018/12/24 8:24:58 AM
RESOURCE PACK
86
LONG QUESTIONS
1.1 A physical quantity which has magnitude (size) and direction . [CL1] (2)
1.2 e sum of two or more vectors. [CL1] (2)
1.3
Correct vectors placed tail-to-head.
Correct resultant.
R
400 N
300 N
ϴ
[CL2] (2)
1.4
R 300 400
222
=+
NR 250 000 500
==
,tan
300
400
53 1°
1
i
==
-
ak
NR 500
=
at 53,1° above the horizontal axis [CL2] (4)
2.1 One TICK for each of the forces shown in their correct places and if the 45° is not
shown take o one mark.
45˚
50 N
40 N
30 N
90 N
[CL2] (4)
2.2
,coscos NFF 90 45 63 64°
x
{ {
i
== =
to the le
,sinsin NFF 90 45 63 64°
y
{{
i
== =
downwards [CL2] (6)
2.3
,,NR 30 50 63 64 83 64
x
= +-- =-
therefore
,NR 83 64
x
=
to the le
,,NR 40 63 64 23 64
y
=+ -=-
therefore
,NR 23 64
y
=
downwards
,,R 83 64 23 64
222
=+
,NR 86 92 {
=
,
,
,tan
83 64
23 64
15 8°
1
{
i
==
-
cm
,NR 86 92
=
at 15,8° below the horizontal axis and to the le [CL3] (5)
Gr11_Physical_Science_Term1_Resource_Book.indb 86 2018/12/24 8:25:02 AM
87
ASSESSMENTS
3.1 e change in position of a body [CL1] (2)
3.2 One mark for each correct vector
Correct resultant (−1 any error)
30 km
40 km
60 km
60 km
R
[CL2] (5)
3.3
km thereforekmwestRR40 60 20 20
xx
{
=+ -=-=
km thereforekmsouthRR30 60 30 30
yy
{
=+ -=-=
One mark for each correct vector
Correct resultant (−1 any error)
ϴ
30 km 30 km
20 km
20 km
R
[CL3] (5)
3.4
R 20 30
222
{
=+
,kmR 36 06
{
=
,tan
20
30
56 3°
1
{
i
==
-
ak
,,
km on abearingR 36 06 213 7
° {
=
[CL3] (4)
4.1 correct force F
correctly labelled components F
x
, F
y
F = 500 N
F
y
F
x
30˚
[CL2] (3)
4.2
,coscos NFF 500 30 433 01°
x
{ {
i
== =
[CL2] (2)
4.3
sinsin NFF 500 30 250°
y
{ {
i
== =
[CL2] (2)
4.4 Friction in the horizontal plane
Weight and the normal force in the vertical plane [CL4] (3)
5.1 A vector has both magnitude and direction , whereas a scalar has
magnitudeonly [CL1] (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 87 2018/12/24 8:25:07 AM
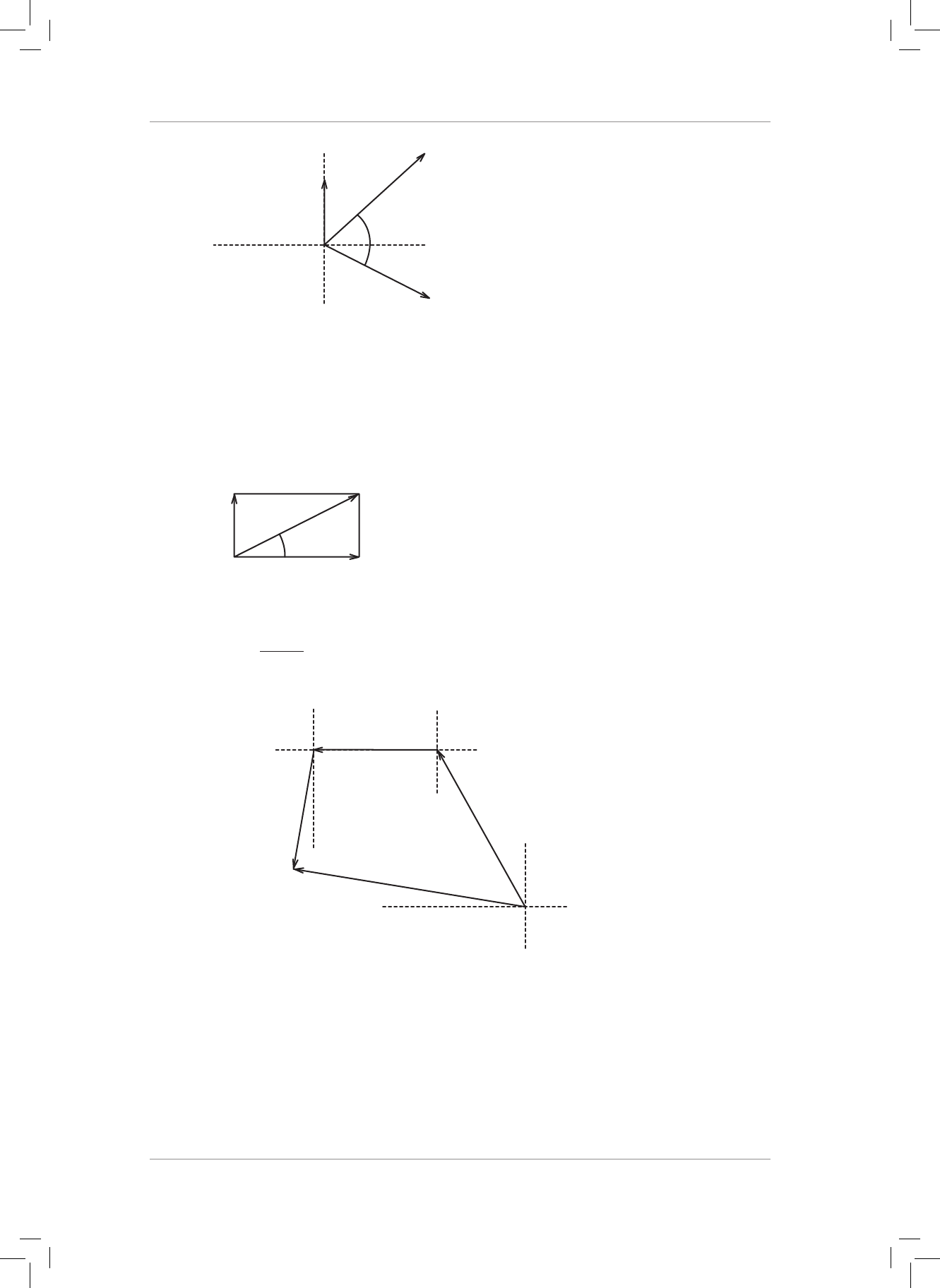
RESOURCE PACK
88
5.2
F = 35 N
1
F = 65 N
2
F = 50 N
3
30˚
45˚
[CL2] (3)
5.3
,coscos NrightFF 65 45 45 96
°
x22
{
==
,sinsin NupFF 65 45 45 96
°
y22
{
i
== =
, coscos NrightFF 50 30 43 30°
x33
{
i
== =
sinsin NdownFF 50 30 25°
y33
{
i
== =
,, , NrightR 45 96 43 30 89 26
x
{
=+ +=
,,
NupR 35 45 96 25 55 96
y
{
=+ +-=
(−1 No diagram OR incorrect diagram)
ϴ
y
R
R
R
x
,,R 89 26 55 96
222
=+
,NR 105 35 {
=
,
,
,tan
89 26
55 96
32 1°
1
{
i
==
-
cm
, ,Natabove thehorizontaland to therightR 105 35 32 1
° {
=
[CL3] (9)
6.1
ϴ
F =400 N (4cm)
3
F =400 N (4cm)
F =600 N (6cm)
R
2
90˚
30˚
10˚
1
,cmR 78 {
=
9°i
=
NbearingR 780 279°{ {
=
[CL3] (10)
6.2 F
4
= 780 N bearing 099°
F
4
should be equal in magnitude to R but opposite in direction. [CL4] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 88 2018/12/24 8:25:13 AM
89
ASSESSMENTS
Topic 2: Newton’s Laws and
Application of Newton’s Laws
QUESTIONS
MULTIPLE CHOICE
1. Which ONE of the following physical quantities is a measure of the inertia of a body?
A Mass
B Energy
C Velocity
D Acceleration (2)
2. e magnitude of the gravitational force exerted by one body on another body
is F. When the distance between the centres of the two bodies is doubled, the
magnitude of the gravitational force, in terms of F, will now be …
A
F
4
1
B
F
2
1
C
F2
D
F4
(2)
3. Two forces, F and T, are applied on a crate lying on a frictionless, horizontal
surface, as shown in the diagram below. e magnitude of force T is greater than
that of force T.
FT
e crate will …
A accelerate towards the right.
B accelerate towards the le.
C move at a constant speed towards the right.
D move at a constant speed towards the le. (2)
4. A person stands on a bathroom scale that is calibrated in newton, in a stationary
elevator. e reading on the bathroom scale is
w
. e elevator now moves with
a constant upward acceleration of
g
3
1
, where
g
is the gravitational acceleration.
What will the reading on the bathroom scale be now?
A
w
3
1
B
w
4
3
C
w
3
4
D
w
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 89 2018/12/24 8:25:16 AM
RESOURCE PACK
90
5. A laptop rests on a table. According to Newton’s 3rd law, what is the reaction force
to the weight of the laptop?
A e upward force of the table on the laptop.
B e upward force of the laptop on the Earth.
C e downward force of the Earth on the laptop.
D e normal force on the laptop. (2)
6. A person stands on a scale in an elevator. He notices that the scale reading is
lower than his usual weight. Which of the following could possibly describe the
motion of the elevator?
A It is moving down at constant speed.
B It is moving down and slowing down.
C It is moving up and slowing down.
D It is moving up and speeding up. (2)
7. e diagram below shows a block of mass M
2
initially at rest on a smooth,
horizontal table. Another block of mass M
1
is attached to it by a light inextensible
cord that passes over a frictionless pulley. When the block M
2
is released, it
accelerates with an acceleration a. What will be the acceleration of block M
2
if the
mass of block M
1
is doubled?
e acceleration is:
A equal to a.
B greater than a and less than 2a.
C equal to 2a.
D greater than 2a.
(2)
LONG QUESTIONS
1. Two blocks of masses 15 kg and 4 kg respectively are connected by a light
inextensible string, P. A second light inextensible string, Q, attached to the 4 kg
block, runs over a light frictionless pulley. A constant horizontal force of 280 N
pulls the second string as shown in the diagram below. e magnitudes of the
tensions in P and Q are T
1
and T
2
respectively. Ignore the e ects of air resistance.
15 kg
Q
PT
1
T
2
280 N
4 kg
Gr11_Physical_Science_Term1_Resource_Book.indb 90 2018/12/24 8:25:17 AM
ASSESSMENTS
1.1 State Newton’s Second Law of Motion in words. (2)
1.2 Draw a labelled free-body diagram indicating ALL the forces acting on the
4kg block. (3)
1.3 Calculate the magnitude of the tension T
1
in string P. (6)
1.4 When the 280 N force is replaced by a sharp pull on the string, one of the
two strings break. Which ONE of the two strings, P or Q, will break? Explain
briey. (2)
[13]
2.1 Two blocks of mass X kg and 2 kg respectively are connected by a light,
inextensible string. e string runs over a light, frictionless pulley, as shown in
the diagram below. e blocks are stationary.
X kg
2 kg
2.1.1 State Newton’s THIRD law in words. (2)
2.1.2 Calculate the tension in the string. (3)
e coecient of static friction (
s
) between the unknown mass X and the surface
of the table is 0,15.
2.1.3 Calculate the minimum value of mass X that will prevent the blocks from
moving. (5)
e block of unknown mass X is now replaced with a block of mass 4,5kg. e
2 kg block now accelerates downwards. e coecient of kinetic friction (
k
)
between the 4,5 kg block and the surface of the table is 0,10.
2.1.4 Calculate the magnitude of the acceleration of the 4,5 kg block. (5)
2.2 A small hypothetical planet A has a mass of 4,5 × 10
20
kg and a radius of 450 km.
Calculate the gravitational force (weight) that planet A exerts on a 800 kg car on
this planet’s surface. (4)
[19]
Gr11_Physical_Science_Term1_Resource_Book.indb 91 2018/12/24 8:25:21 AM
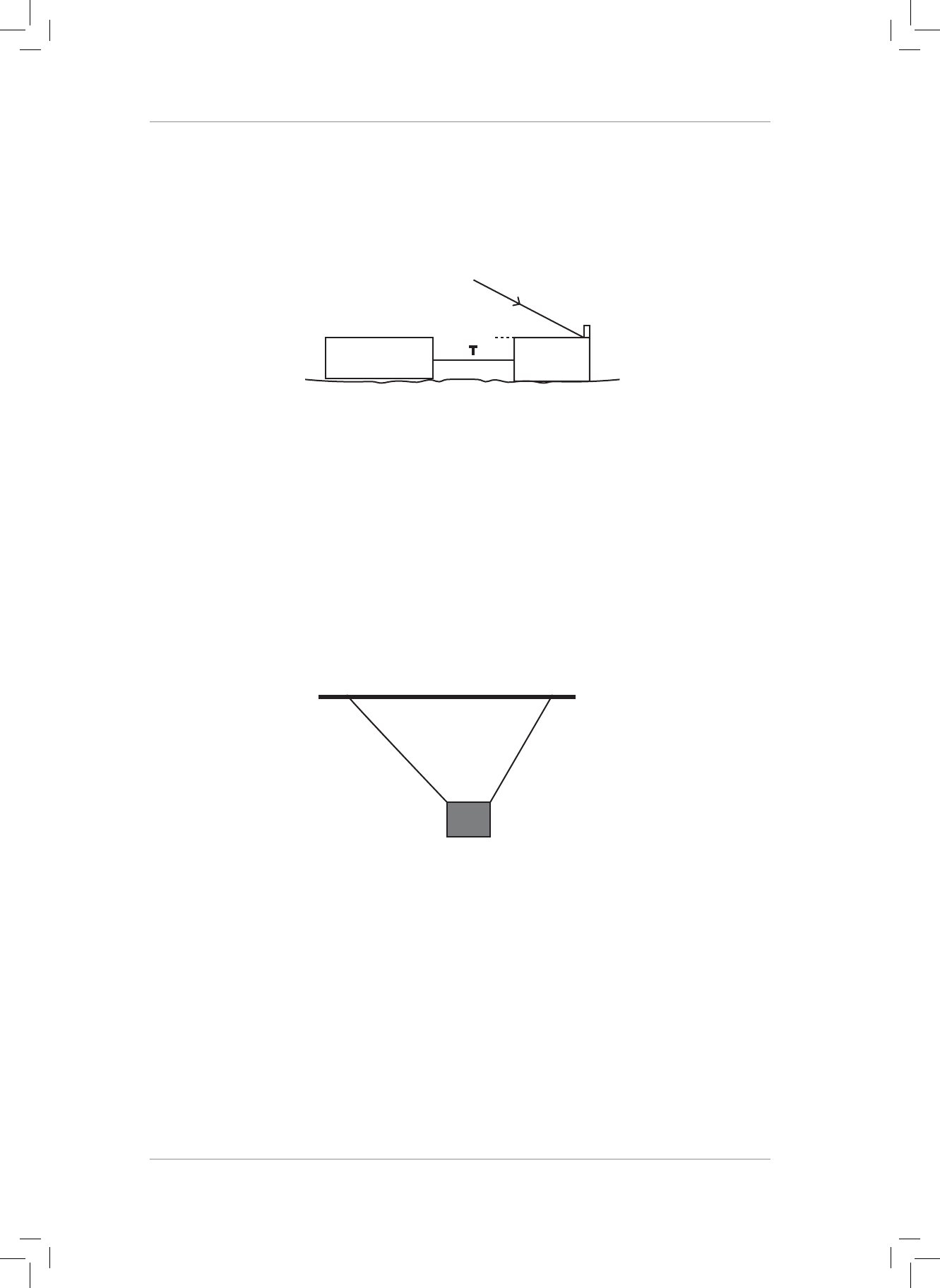
RESOURCE PACK
92
3. A learner constructs a push toy using two blocks with masses 2 kg and 4 kg
respectively. e blocks are connected by a massless, inextensible cord.
e learner then applies a force of 30 N at an angle of 25° to the 2 kg block by
means of a light rigid rod, causing the toy to move across a at, rough, horizontal
surface, as shown in the diagram below.
4 kg
30 N
25˚
2 kg
T
e coecient of kinetic friction (
k
) between the surface and each block is 0,20.
3.1 State Newton’s Second Law of Motion in words. (2)
3.2 Calculate the magnitude of the kinetic frictional force acting on the 4 kg block. (3)
3.3 Draw a labelled free-body diagram showing ALL the forces acting on the
2kg block. (5)
3.4 Calculate the magnitude of the:
3.4.1 kinetic frictional force acting on the 2 kg block. (3)
3.4.2 tension in the cord connecting the two blocks. (5)
[21]
4. A block of unknown mass hangs stationary from the ceiling by means of two
strings as shown in the diagram below.
40˚
String
1S
tring 2
60˚
e tension in string 1 is 450 N.
4.1 Are the forces acting on the block in equilibrium? Explain your answer. (3)
4.2 Determine the magnitude of the horizontal component of the tension in
string 1. (2)
4.3 Calculate the magnitude of the tension in string 2. (3)
4.4 Calculate the mass of the block. (5)
[13]
Gr11_Physical_Science_Term1_Resource_Book.indb 92 2018/12/24 8:25:23 AM
93
ASSESSMENTS
5. An empty li of mass 800 kg is accelerated upwards at 2 ms
-2
from the ground
oor of an oce building.
5.1 Draw a labelled free-body diagram of the accelerating li, showing the forces
acting on the li. Your diagram should reect the relative sizes of the forces. (3)
5.2 State Newton’s second law. (2)
5.3 Calculate the tension in the li cable while the empty li is accelerated
upwards. (4)
5.4 e li cable is able to withstand a maximum tension of 18 kN. Determine
the maximum cargo mass that the li can carry while accelerating upwards
at 2 ms
-2
. (5)
[14]
6. Block X has a mass of 4 kg and Block Y has a mass of 2 kg. Block X is placed on
a rough table that has a coecient of static friction of 0,18. Block X and Block Y
are joined by a light, inextensible string over a frictionless pulley and Block Z is
placed on top of Block X as shown in the diagram below.
X = 4 kg
Y = 2 kg
Z
e system, as shown in the diagram, is only just at rest and on the limit of sliding.
6.1 Determine the tension in the rope joining Block X and Block Y while the
system is atrest. (2)
6.2 Dene frictional force. (2)
6.3 State the magnitude of the frictional force required for Block X to be at rest. (2)
6.4 Calculate the minimum mass of Block Z to stop Block X from sliding. (5)
Block Z is removed and the system starts accelerating.
6.5 State Newton’s second law. (2)
6.6 Draw a labelled, free-body diagram for Block Y while it is accelerating. (2)
e magnitude of the acceleration of the system is 2,5 ms
–2
.
6.7 Calculate the tension in the rope joining the blocks. (3)
6.8 Calculate the frictional force acting on Block X while it is accelerating. (3)
6.9 State two reasons why the frictional force between Block X and the surface is
smaller than the value stated in Question 6.3. Briey explain your answer. (4)
[25]
Gr11_Physical_Science_Term1_Resource_Book.indb 93 2018/12/24 8:25:24 AM

RESOURCE PACK
94
7. e photograph shows the result of an incident
of emergency braking carried out by a truck
driver. He was driving a truck (mass 10800kg)
at 80kmh
–1
while carrying a granite block (mass
160000kg) in the trailer. He braked suddenly
to avoid hitting a child who crossed the road
in front of him. It was agreed aer the incident
that the driver was in no way to blame. (He was
completely innocent of any wrong doing).
7.1 With reference to one of Newton’s Laws, explain the behaviour of the granite
block. (4)
7.2 Convert 80 kmh
–1
to ms
–1
. (3)
e skid marks of the truck on the road were 38,2 m in length from the time the
driver slammed on the brakes to when the truck stopped.
7.3 Calculate the acceleration of the truck. (4)
7.4 Calculate the magnitude of the braking force exerted on the truck while it
was brought to a standstill. (3)
7.5 Suggest a way in which the trucking company can prevent accidents of this
kind from happening when the trucks carry a heavy load, such as blocks of
granite, in the future. (1)
[15]
8. While an astronaut (mass 75 kg) works in Space at a distance of 15 m from his
spaceship, his power pack fails to work. e pack has a mass 25 kg. e astronaut
knows that now his only way of getting back to his spaceship is to push his pack
away from him.
8.1 State an appropriate law of physics, and use it to explain how the astronaut is
able to return to his spaceship by pushing his pack away from him. (6)
8.2. State Newton’s Law of Universal Gravitation. (3)
8.3. When the astronaut gets to Mars he feels much lighter than he did on Earth
because the acceleration due to gravity on Mars is 3,5 ms
−2
. e mass of
Mars is 6,4 × 10
23
kg. Calculate the radius of Mars in m. (5)
8.4 e astronaut, wearing his spacesuit and life support systems, jumps
vertically upwards from the surface of Mars. Will the astronaut be able to
jump higher, from the same initial velocity (while wearing the same kit)
on Mars than on Earth? Justify your answer, by considering an appropriate
formula. (5)
[19]
Gr11_Physical_Science_Term1_Resource_Book.indb 94 2018/12/24 8:25:24 AM

95
ASSESSMENTS
MARKING GUIDELINES
MULTIPLE CHOICE
1. A (Related to the denition) [CL1] (2)
2. A
FG
r
mm
2
12
=
FG
r
mm
G
r
mm
G
r
mm
F
2
4
4
1
4
1
new
2
12
2
12
2
12
==
==
^
`
h
j
[CL3] (2)
3. B Since F is greater than T, the net force acts to the le, therefore the
acceleration isalso le. [CL2] (2)
4. C
Fma
net
=
Fw ma
-=
Fmgm g
3
1
-=
a
k
Fmgm g
3
1
=+
a
k
Fm
gw
3
4
3
4
==
[CL4] (2)
5. B Weight of laptop = Downward force of Earth on laptop.
Reaction force = Upward force of laptop on Earth. [CL2] (2)
6. C If the reading on scale (the upward force) is less than his weight (the
downward force), the net force on him acts downwards. e direction
of his acceleration is alsodown. [CL3] (2)
7. B Initially
a
MM
M
12
1
=
+
g
When M
1
is doubled,
a
MM
M
2
2
'
12
=
+
g
erefore
a
a
MM
Mg
Mg
MM
MM
MM
2
2
2
2
'
12
1
1
12
12
12
#
=
+
+
=
+
+
^h
is value is greater than 1 and less than 2. [CL 4] (2)
LONG QUESTIONS
1.1 When a net force is applied to an object of mass, m, it accelerates in the direction
of the net force. e acceleration, a, is directly proportional to the net force and
inversely proportional to the mass. [CL1] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 95 2018/12/24 8:25:28 AM
RESOURCE PACK
96
1.2
Weight w
Tension in Q (T
2
)
Tension in P (T
1
)
[CL3] (3)
1.3 Choose up as positive:
4 kg: 15 kg:
Fma
net
=
Fma
net
=
TW
Ta
4
kg24 1
{
--=
^h
TW a15
kg115
{
-=
^h
, Ta280392 4
1
--=
Ta147 15
1
-=
, Ta2408 4
1
-=
Ta147 15
1
{
=+
(ii)
,Ta240 84
1
{
=-
(i)
Set equation (i) equal to equation (ii)
, aa2408 4 147 15
-= +
, a93 819
=
.
,msa 494
2
`
{
=
-
,,
,,
NTa240 84 240 84494 221 04
1
{
=-=- =
^h
[CL3] (6)
1.4 P Since the 15 kg mass has the greater inertia [CL4] (2)
2. 2.1.1 When object A exerts a force on object B, object B simultaneously exerts an
oppositely directed force of equal magnitude on object A. [CL1] (2)
2.1.2
,,NupTw 298196
kg2
#
{{{
== =
[CL2] (3)
2.1.3
,Nf 19 6
max
s
{
=
to the le
,,,fNmg
XX
01598147
max
sss
{{ {
nn
==
==
^^ ^^hhhh
,,X19 6147
=
,kgX 13 33
=
[CL3] (5)
2.1.4 Choose right and down as positive:
4,5 kg: 2 kg:
Fma
net
=
Fma
net
=
,Tf a45
k
{
-=
WTa2
kg2
{
-=
,(,) ,Ta01 44 145
-=
^h
, Ta19 62
-=
,,Ta44145
-=
,Ta19 62{
=-
(ii)
,,Ta44145 {
=+
(i)
Set equation (i) equal to equation (ii)
,, ,aa44145196 2
+=-
,,a65 15 19
=
s {
$
,ma 234
2
=
-
[CL3] (5)
One mark for each
correct force which
is correctly labelled.
Gr11_Physical_Science_Term1_Resource_Book.indb 96 2018/12/24 8:25:36 AM
97
ASSESSMENTS
2.2
FG
r
mm
2
12
=
F
=
,
,
66710
450 10
45 10 800
11
3
2
20
#
#
#
{
{
-
^
^
^
^
h
h
h
h
,N
F 118 58
{
=
towards the centre of the planet. [CL3] (4)
“Force of attraction” does not specify the direction of the force. e direction of
the force is towards the centre of the planet. Attraction describes the type of force
that acts on the car.
3.1 When a net force is applied to an object of mass, m, it accelerates in the direction
of the net force. e acceleration, a, is directly proportional to the net force and
inversely proportional to the mass. [CL1] (2)
3.2
,,,. NfN020498 784
kk
{{ {
n
== =
^^^hh h
[CL2] (3)
3.3
For each correctly
labelled force.
−1 If the angle is not
shown.
Weight w
Applied force F = 30 N
65°
Tension T
Friction f
k
Normal N
[CL2] (5)
3.4.1
,sinsin NFF25 30 25 12 68
°°
y
{
== =
downwards
,,
,N
FFW 12 68 19 63228
down y
{
=+ =+=
downwards
erefore:
,NN 32 28
=
upwards
,, ,. NfN0203228646
kk
{
n
== =
^^hh
[CL3] (3)
3.4.2
,coscos NFF 25 30 25 27 19
°°
x
{
== =
^h
to the right
Choose right as positive:
2 kg: 4 kg:
Fma
net
=
Fma
net
=
FTfa2
xk
--=
^h
Tf a4
k
-=
^h
,,Ta27 19 6462{
-- =
,Ta7844{
-=
, Ta20 73 2
-=
,Ta7844
=+
(ii)
,Ta20 73 2 {
=-
(i)
Set equation (i) equal to equation (ii)
,,aa20 73 2784 4
-= +
, a12 89 6
=
s
$
,ma 214
2
=
-
,,
,,
NTa20 73 2207322141645
{
=-=- =
^h
[CL3] (5)
4.1 Yes . e block remains at rest, therefore the net force is zero. [CL2] (3)
4.2
,coscos NTT 40 450 40 344 72
°°
x11
{ {
== =
[CL2] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 97 2018/12/24 8:25:43 AM

RESOURCE PACK
98
4.3
coscosTT T60 60°°
x22 2
==
TT
xx12
=
(net force in horizontal plane is zero)
,c
osT344 72 60
°
2
{ {
=
,
,
cos
NT
60
344 72
689 44
°
2
{
==
[CL3] (3)
4.4
4,
sinsin NTT40 50 40 289 25
°°
y11
{
== =
upwards
,,sinsin NTT60 689 44 60 597 07°°
y22
{
== =
upwards
wT T
yy12
=+
,,
,N
w 289 25 597 07 886 32
{
=+=
[CL3] (5)
,
,
,k
gm
g
w
98
886 32
90 44
{
{
== =
5.1
Weight w
Tension T
T is greater than w
[CL2] (3)
5.2 When a net force is applied to an object of mass, m, it accelerates in the direction
of the net force. e acceleration, a, is directly proportional to the net force and
inversely proportional to the mass. [CL1] (2)
5.3 Choose up as positive.
Fma
net
=
()Tw 800 2 {
-=
^h
T 7 840 1 600{
-=
NT 9 440 {
=
upwards [CL3] (4)
5.4 Choose up as positive
Fma
net
=
Tw m2
-=
()mg m18 000 2{{{
-=
, mm18 000 98 2
-=
, m18 000 11 8
=
,kgm 1 525 42 {
=
Cargo mass = 1525,42 – 800 = 725,42 kg [CL4] (5)
6.1
,,
Nw 298196
kg2
==
^^hh
erefore:
,NT 19 6
{{
=
[CL2] (2)
6.2 e force that opposes the motion of an object and acts parallel to the surface
the object is in contact with [CL1] (2)
6.3
,Nf 19 6
{{
=
[CL2] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 98 2018/12/24 8:25:53 AM
99
ASSESSMENTS
6.4
fN
max
ss
n
=
,, (, )m19 6018 98{{ {
=
^^hh
,kgm 11 11 {
=
,,kgm 11 11 4711
z
{
=-=
[CL3] (5)
6.5 When a net force is applied to an object of mass, m, it accelerates in the direction
of the net force. e acceleration, a, is directly proportional to the net force and
inversely proportional to the mass. [CL1] (2)
6.6
Weight w
Tension T
[CL2] (2)
6.7 Choose down as positive. Working with the net force on Y:
Fma
net
=
(,)wT 225
{ {
-=
^h
, T19 65
-=
,N
T 14 6
{
=
upwards on the 2 kg block [CL3] (3)
6.8
Fma
net
=
(,)Tf 425
-=
^h
, f14 610
-=
, Ntothe leftf 46 { {
=
[CL3] (3)
6.9 e coecient of kinetic friction is always less than the coecient of static
friction.
e weight of the block has decreased, therefore the normal force has decreased.
f
is directly proportional to the normal force N. [CL4] (4)
7.1 e granite block continues to move at its initial speed (of 80 kmh
−1
) when
the truck stops because there is no net force acting on the block. According to
Newton’s First Law, the block continues in its state of constant velocity until it
crashes into the cab of the truck (until a net force acts on it). [CL 3] (4)
7.2 80 kmh
−1
=
60 60
80 1000
#
#
{
{
= 22,22 ms
−1
[CL 2] (3)
7.3 v
i
= 22,22 ms
−1
vf = 0 ms
−1
∆x = 38,2 m
v
f
2
= v
i
2
+ 2a∆x
0 = (22,22)
2
+ 2a(38,2)
a = − 6,46 ms
−2
= 6,46 ms
−2
backwards [CL 3] (4)
7.4 F
net
= ma
= (10800 + 160000) × 6,46
= 1103368 N (or 1,10 × 10
6
N) [CL 4] (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 99 2018/12/24 8:25:58 AM

RESOURCE PACK
7.5 e block of granite should be held rmly in place in the trailer (restrained by
straps to prevent it from moving forwards or backwards while the truck is in
motion. [CL 3] (1)
8.1 When a body A exerts a force on body B, body B simultaneously exerts a force of
the same magnitude but in the opposite direction on body A. (Newton’s 3rd
Law. ONLY 1 MARK IF IT IS JUST STATED AS THE LAW (WITHOUT THE
LAW STATED IN WORDS).
e astronaut exerts force on the power pack, pushing it away from him. e
power pack exerts force on the astronaut pushing him in the opposite direction.
is push will help him reach the spaceship because there is no air resistance
(or frictional forces) acting on him in Space. Once he starts moving towards
the spaceship he will continue to move at constant velocity. [CL 4] (6)
8.2 ere exists a force of attraction between any two objects in the universe. e
force is directly proportional to the product of their masses and inversely
proportional to the square of the distance between their centres. [CL 1] (3)
8.3
.
,
(, )(,)
,,ms
g
r
Gm
r
r
38
667106410
1 1234 10 10610
2
2
11 22
12
62
##
##
{
{
{
{
{
=
=
==
-
-
[CL 3] (5)
8.4 He will jump higher on Mars [because g
earthg
> g
moon
]
v
f
2
= v
i
2
+ 2 a∆y
On the Mars 0 = v
i
2
+ 2(3,8) ∆y therefore ∆y = v
i
2
/ (7,6)
On the Earth 0 = v
i
2
+ 2(9,8) ∆y therefore ∆y = v
i
2
/ (19,6)
erefore for the same launch speed (v
i
) he jumps higher on Mars. [CL 4] (5)
Gr11_Physical_Science_Term1_Resource_Book.indb 100 2018/12/24 8:25:58 AM
ASSESSMENTS
Topic 3: Atomic Combinations
(Molecular Structure)
QUESTIONS
MULTIPLE CHOICE
1. Which chlorine compound has bonding that can be described as ionic with some
covalent character?
A NaC
B MgC
2
C AC
3
D SiC
4
(2)
2. Which molecule has only six bonding electrons?
A C
2
H
4
B C
2
F
6
C H
O
D NF
3
(2)
3. What set of data would hydrogen sulde, H
2
S, be expected to have?
Number of bonding pairs Shape
A 1 Trigonal planar
B 2 Pyramidal
C 2 Angular
D 3 Pyramidal
(2)
4. Which ONE of the following best describes the bond formed between an H
+
ion
and the NH
3
molecule to form an NH
4
+
ion?
A covalent bond
B dative covalent bond
C ionic bond
D hydrogen bond (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 101 2018/12/24 8:25:59 AM
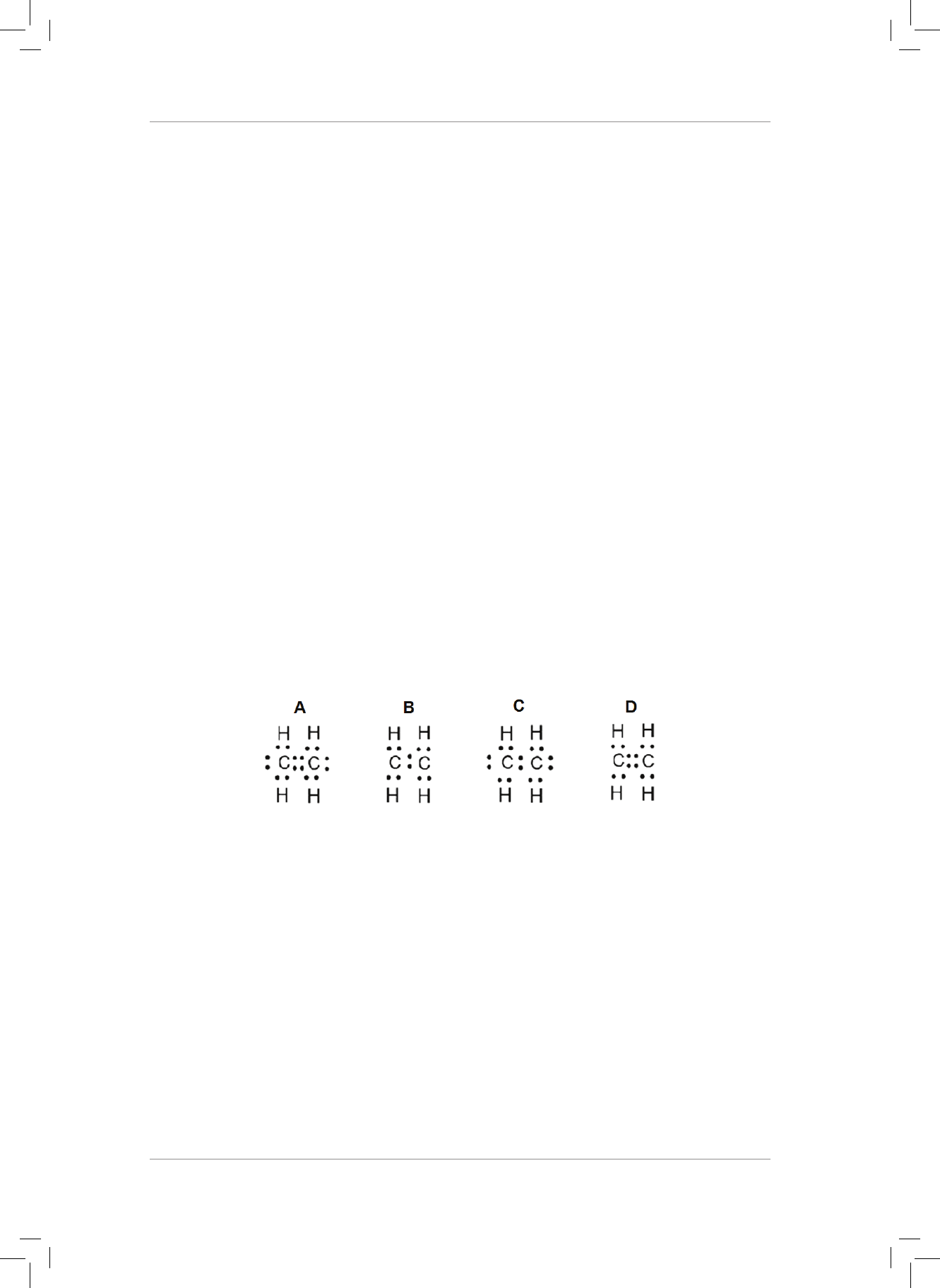
RESOURCE PACK
5. Refer to the following list of substances and the electronegativities provided on
the Periodic Table.
CO
2
NH
3
H
2
O N
2
HF
Which of the following lists all the non-polar molecules from the above list?
A N
B N
2
and NH
3
C N
2
and CO
2
D N
2
, CO
2
and NH
3
(2)
6. Which of the following pure substances will conduct electric current when
molten (in the liquid phase)?
Pb PbBr
2
C
6
H
12
O
6
(glucose) CH
3
CH
2
OH (alcohol)
A Pb and PbBr
2
B Pb and CH
3
CH
2
OH (alcohol)
C Pb, PbBr
2
and CH
3
CH
2
OH (alcohol)
D All of these substances (2)
7. A dative covalent bond forms when…
A water decomposes.
B ionic compounds dissociate in water.
C hydronium ions are produced.
D hydroxide ions are produced. (2)
8. Which of one of the following Lewis structures correctly represents a molecule of
C
2
H
4
?
1.5 Refer to the following list of substances and the electronegativities provided on the Periodic
Table.
CO
2
NH
3
H
2
O N
2
HF
Which of the following lists all the non-polar molecules from the above list?
A N
2
B N
2
and NH
3
C N
2
and CO
2
D N
2
, CO
2
and NH
3
(2)
1.6 Which of the following pure substances will conduct electric current when molten (in its liquid
phase)?
Pb PbBr
2
C
6
H
12
O
6
(glucose) CH
3
CH
2
OH (alcohol)
A Pb and PbBr
2
B Pb and CH
3
CH
2
OH (alcohol)
C Pb, PbBr
2
and CH
3
CH
2
OH (alcohol)
D All of these substances (2)
1.7 A dative covalent bond forms when…
A water decomposes.
B ionic compounds dissociate in water.
C hydronium ions are produced.
D hydroxide ions are produced. (2)
1.8 Which of one of the following Lewis structures correctly represents a molecule of C
2
H
4
?
(2)
1.9 Consider the Lewis structure of a compound shown below:
Which ONE of the following is correct?
Name of element X Name of element Y Molecular shape of
compound
A chlorine sulfur angular
B
oxygen chlorine angular
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 102 2018/12/24 8:26:00 AM
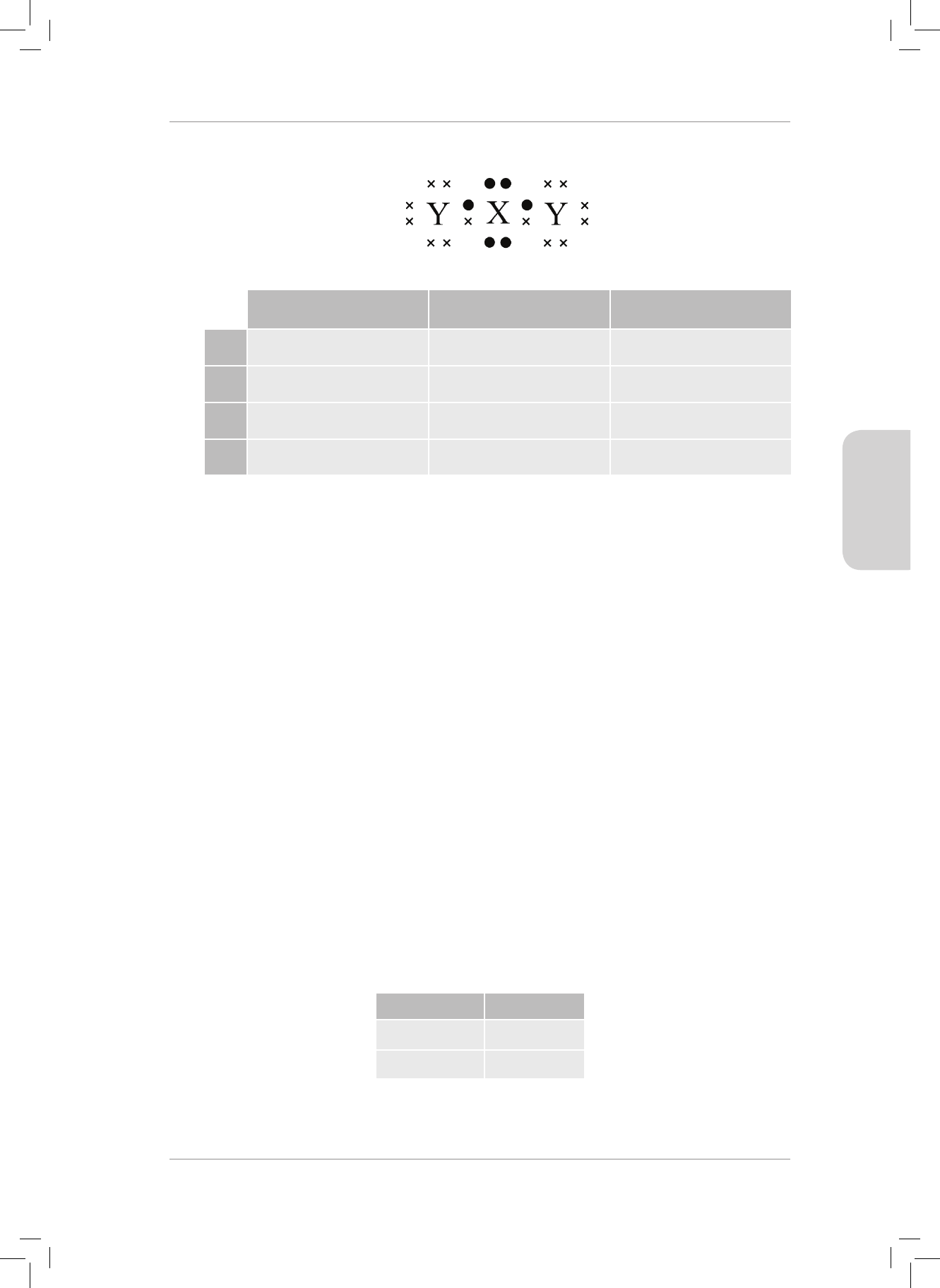
ASSESSMENTS
9. Consider the Lewis structure of a compound shown below:
Which ONE of the following is correct?
Name of element X Name of element Y Molecular shape of
compound
A
chlorine sulfur angular
B
oxygen chlorine angular
C
chlorine oxygen linear
D
sulfur chlorine linear
(2)
10. Which of the following substances has polar covalent bonds between its atoms,
but its molecule is non-polar?
A H
2
O
B H
C HC
D CC
4
(2)
LONG QUESTIONS
1.1 Study the following list of substances:
Hg I
2
SF
6
KI PC
3
Ne C H
2
O
For each statement below choose a substance that displays those properties. You
can choose a substance once, more than once or not at all.
1.1.1 A substance that conducts electricity when molten but not when solid. (1)
1.1.2 A substance that forms a covalent crystal lattice. (1)
1.1.3 A substance that consists of non-polar molecules containing polar bonds. (1)
1.1.4 Its molecules have two lone pairs on the central atom. (1)
1.1.5 A substance which has delocalised electrons. (1)
1.1.6 Its molecules have a non-ideal shape. (1)
1.1.7 A substance which consists of monatomic molecules. (1)
1.2 Refer to the bond energies supplied below:
A B
C−C C=C
-1
-1
Gr11_Physical_Science_Term1_Resource_Book.indb 103 2018/12/24 8:26:01 AM
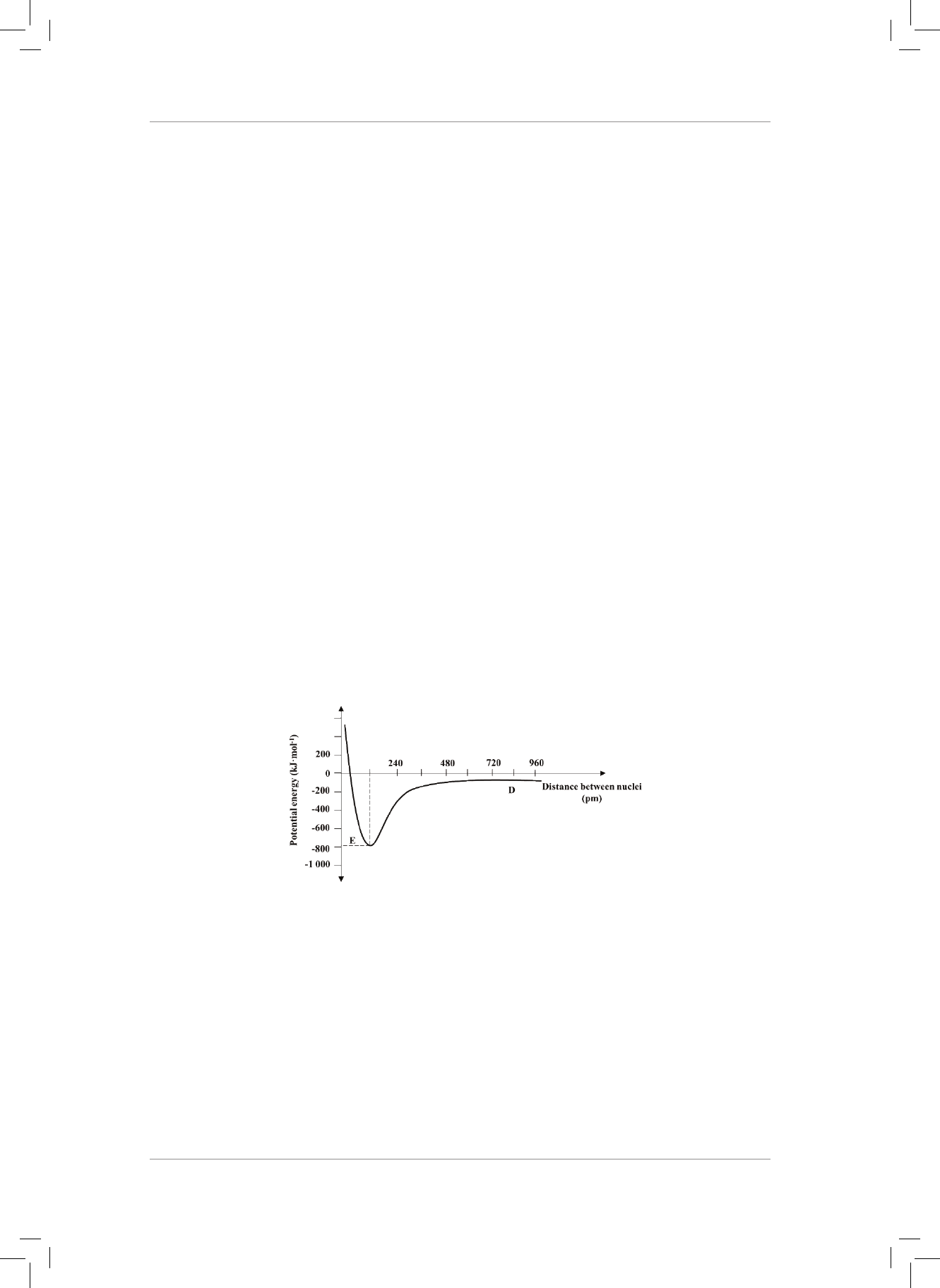
RESOURCE PACK
1.2.1 What information does the bond energy provide? (1)
1.2.2 Which of A or B will have the shorter bond length? (1)
1.2.3 Explain why the bond energy in B is not double the bond energy in A. (2)
[11]
2.1 South Africa has comparable greenhouse gas emissions to many industrialised
countries. e main air pollutants generated by South African industries are
carbon dioxide (CO
2
), methane (CH
4
), carbon monoxide (CO), sulfur dioxide
(SO
2
), the oxides of nitrogen (NO
2
, NO and N
2
O) and ammonia (NH
3
).
2.1.1 Draw Lewis structures to represent:
a) carbon dioxide. (2)
b) methane. (2)
2.1.2 De ne the term “electronegativity”. (2)
2.1.3 By means of a suitable calculation describe the type of bonding that occurs
in a molecule of sulfur dioxide. (2)
2.1.4 e chemical bonds within the methane molecule are polar covalent
and yet methane is known to be a non-polar molecule. Explain how this
phenomenon comes about. Show the polarity of the molecule with the use
of a Lewis diagram. (4)
2.1.5 Of the pollutants listed above, choose
a) one trigonal pyramidal
b) one tetrahedral and
c) one linear molecule. (3)
2.2 e graph below shows the change in energy that takes place when an oxygen
atom approaches a carbon atom during the formation of a C=O bond.
2.1. South Africa has comparable greenhouse gas emissions to many industrialised
countries. The main air pollutants generated by South African industries are carbon
dioxide (CO
2
), methane (CH
4
), carbon monoxide (CO), sulfur dioxide (SO
2
), the oxides
of nitrogen (NO
2
, NO and N
2
O) and ammonia (NH
3
).
2.1.1. Draw Lewis structures to represent:
a) carbon dioxide.
(2)
b) methane.
(2)
2.1.2. Define the term “electronegativity”.
(2)
2.1.3. By means of a suitable calculation describe the type of bonding that occurs in
a molecule of sulfur dioxide.
(2)
2.1.4. The chemical bonds within the methane molecule are polar covalent and yet
methane is known to be a non-polar molecule. Explain how this phenomenon
comes about. Show the polarity of the molecule with the use of a Lewis
diagram.
(4)
2.1.5. Of the pollutants listed above, choose
a) one trigonal pyramidal
b) one tetrahedral and
c) one linear molecule.
(3)
2.2. The graph below shows the change in energy that takes place when an oxygen atom
approaches a carbon atom during the formation of a C=O bond.
2.2.1. Define the term ‘bond length’.
(2)
2.2.2. From the graph, write down the:
(a) bond length, in pm, of the C=O bond.
(1)
(b) bond energy, in kJ·mol
-1
needed to break the C=O bond.
(2)
(c) Name the potential energy represented by E.
(1)
2.2.3. How will the bond length of an S=O bond compare to that of the C=O bond?
Write down EQUAL TO, SHORTER THAN or LONGER THAN. Give a reason
for your answer.
(2)
2.2.4. What can be said about the forces between the two atoms at point D?
(2)
[25]
3. 3.1 CO
2
(g), SO
2
and CO(g) are some of the air pollutants in the South African atmosphere.
3.1.1 CO has a dative covalent bond. Draw the Lewis structure for a carbon monoxide molecule
and use it to help explain the idea of a dative covalent bond. (3)
2.2.1 De ne the term ‘bond length’. (2)
2.2.2 From the graph, write down the:
(a) bond length, in pm, of the C=O bond. (1)
(b) bond energy, in kJ·mol
-1
needed to break the C=O bond. (2)
(c) Name the potential energy represented by E. (1)
2.2.3 How will the bond length of an S=O bond compare to that of the C=O bond?
Write down EQUAL TO, SHORTER THAN or LONGER THAN. Give a
reason for your answer. (2)
2.2.4 What can be said about the forces between the two atoms at point D? (2)
[25]
Gr11_Physical_Science_Term1_Resource_Book.indb 104 2018/12/24 8:26:02 AM
ASSESSMENTS
3. 3.1 CO
2
(g), SO
2
and CO(g) are some of the air pollutants in the South African
atmosphere.
3.1.1 CO has a dative covalent bond. Draw the Lewis structure for a carbon
monoxide molecule and use it to help explain the idea of a dative
covalent bond. (3)
3.1.2 Explain why you would expect the CO molecule to be polar. (2)
3.1.3 What is the nature of the partial charges on (i.e. the polarity of) the C
and O atoms in the CO molecule? Explain your answer. (2)
3.2 Consider the gases CO
2
and SO
2
.
3.2.1 Refer to Lewis Diagrams and the VSEPR theory. Compare the shapes
of a CO
2
molecule and a SO
2
molecule, both of which contain two
oxygen atoms bonded to one other atom. (4)
3.2.2 Comment on the polarity of both the CO
2
and SO
2
molecules. In each
instance, explain your answer with reference to the molecular shape. (4)
[15]
4. Sulfur forms many compounds by reacting with other elements.
4.1 ree of these compounds are sodium sulde, SOC
2
, SF
2
and two other
uorides.
4.1.1 Draw Lewis diagrams to show the bonding in:
a) sodium sulde (3)
b) SF
2
(2)
4.1.2 ere are two possible ways of drawing the Lewis diagram of SOC
2
.
Draw the two possible Lewis structures. Explain how you arrived at
each of the structures. (5)
4.1.3 Use the valence shell electron pair repulsion (VSEPR) theory to
deduce the shapes of the following molecules:
a) SF
(2)
b) BeC
2
(2)
4.2 Sulfur is in group 16 and period 3 of the Periodic Table.
4.2.1 Dene the terms:
a) Electronegativity (2)
b) Bond polarity (2)
4.2.2 Give reasons for your answers to the next two questions.
Identify the type of bonds that form in
a) SF
2
(3)
b) H
2
S (3)
[24]
Gr11_Physical_Science_Term1_Resource_Book.indb 105 2018/12/24 8:26:02 AM

RESOURCE PACK
5. 5.1 e hydrogen halides (hydrogen uoride, hydrogen chloride, hydrogen
bromide and hydrogen iodide) are important chemicals.
e diagram below represents a molecule of hydrogen chloride.
3.1.2 Explain why you would expect the CO molecule to be polar. (2)
3.1.3 What is the nature of the partial charges on (i.e. the polarity of) the C and O atoms in the
CO molecule? Explain your answer. (2)
3.2 Consider the gases CO
2
and SO
2
.
3.2.1 Refer to Lewis Diagrams and the VSEPR theory. Compare the shapes of a
CO
2
molecule and a SO
2
molecule, both of which contain two oxygen atoms
bonded to one other atom. (4)
3.2.2 Comment on the polarity of both the CO
2
and SO
2
molecules. In each instance, explain
your answer with reference to the molecular shape. (4)
[15]
4. Sulfur forms many compounds by reacting with other elements.
4.1 Three of these compounds are sodium sulfide, SOCl
2
, SF
2
and two other fluorides.
4.1.1 Draw Lewis diagrams to show the bonding in:
a) sodium sulfide (3)
b) SF
2
(2)
4.1.2 There are two possible ways of drawing the Lewis diagram of SOCl
2
. Draw the two
possible Lewis structures. Explain how you arrived at each of the structures. (5)
4.1.3 Use the valence shell electron pair repulsion (VSEPR) theory to deduce the shapes of the
following molecules:
a) SF
2
(2)
b) BeCl
2
(2)
4.2 Sulfur is in group VI and period 3 of the Periodic Table.
4.2.1 Define the terms:
a) Electronegativity (2)
b) Bond polarity (2)
4.2.2 Give reasons for your answers to the next two questions.
Identify the type of bonds that form in
a) SF
2
(3)
b) H
2
S (3)
[24]
5. 5.1 The hydrogen halides (hydrogen fluoride, hydrogen chloride, hydrogen bromide and
hydrogen iodide) are important chemicals.
The diagram below represents a molecule of hydrogen chloride.
5.1.1 What type of particles are represented by the crosses (X)? (1)
Cl
H
x
x
x xx x
x xx x
x
x
x
x
x
x
x
x
5.1.1 What type of particles are represented by the crosses (X)? (1)
5.1.2 What type of chemical bond holds the atoms in this molecule together? (1)
5.2 e relative amount of energy (in kJmol
-1
) required to break the bond in
each of the hydrogen halide molecules is shown below.
H – F 569
H – C 432
H – Br 366
H – I 298
One of the important properties of the hydrogen halides is that they dissolve
in water to form acids. For example, hydrogen chloride reacts with water to
form hydrochloric acid.
To form an acid the bond between the hydrogen and the halogen atoms
must be broken and ions are formed. e stronger the acid the more
molecules that split up to form ions.
5.2.1 Which ion must be formed to make a solution acidic? (1)
5.2.2 Which of the hydrogen halides would you expect to react with water
to form the strongest acid? Explain your answer. (3)
[6]
6. A learner studies uorine and carbon and the bonds that atoms of each can
form with other atoms. Fluorine is a reactive element that can react with water
and with many metals. Carbon is a very special element because it is one of the
elements in the food we eat, the clothes we wear and the fuel in our cars.
6.1 Write down the number of electrons in the valence energy level of an atom of:
6.1.1 uorine. (1)
6.1.2 carbon. (1)
6.2 Describe what is meant by the term ‘covalent bond’. (2)
6.3 Write down the Lewis structure for:
6.3.1 F
2
(2)
6.3.2 HCN (2)
6.4 Write down the name of the type of bond between particles in molecules of:
6.4.1 F
2
(1)
6.4.2 HF (1)
Gr11_Physical_Science_Term1_Resource_Book.indb 106 2018/12/24 8:26:03 AM

ASSESSMENTS
6.5 Write down the molecular shape as predicted by the VSEPR model of:
6.5.1 BF
3
(2)
6.5.2 CH
4
(2)
6.5.3 HF (2)
6.6 e table below shows bond lengths and bond energies for dierent bonds
between two carbon atoms.
Bond Length (pm)
-1
)
A
C − C 154 348
B
C = C
134 614
C
C / C
120 839
6.6.1 Describe the relationship between bond length and bond energy as
shown in the above table. (2)
6.6.2 Which one of the bonds (A, B or C) will be the weakest? Explain. (3)
[20]
Gr11_Physical_Science_Term1_Resource_Book.indb 107 2018/12/24 8:26:03 AM
RESOURCE PACK
MARKING GUIDELINES
MULTIPLE CHOICE
1. B ∆ electronegativity = 3,0 – 1,2 = 1,8 which implies that the bond is
polar covalent. However, Mg is a metal bonding with the non-metal C,
therefore the bond is ionic. is is the compound which is ionic with
some covalent character. [CL3] (2)
2. D Nitrogen has three unpaired electrons which it is able to share with
the three uorine atoms. ere are three bonds around each nitrogen
atoms. Each bond consists of a shared pair of electrons. erefore, this
compound has 6 bonding electrons (six electrons involved in chemical
bonds). [CL2] (2)
3. C ere are two bonding pairs because two hydrogen atoms are attached
to the sulfur atoms. e two lone pairs of sulfur repel each other
strongly so the shape of H
2
S is similar to that of water. It is angular (or
bent). [CL2] (2)
4. B e hydrogen ion bonds with the ammonia molecule by attaching to
the lone pair of electrons. is type of covalent bond where one atom
donates both electrons to form the bond is called a dative covalent
bond. [CL3] (2)
5. C Nitrogen is non-polar because it consists of three non-polar covalent
bonds. CO
2
is non-polar because its molecule is linear, with the eect of
the two polar covalent bonds directed in opposite directions, resulting
in an overall non-polar molecule. [CL2] (2)
6. A Pb is a metal with delocalised electrons to carry charge through the
substance. PbBr
2
in molten form dissociates into its ions (cations and
anions). ese ions are free to carry charge throughout the molten
liquid. [CL2] (2)
7. C Hydronium ions are formed when a hydrogen ion (H
+
ion) attaches
itself to one of the lone pairs of electrons of a water molecule. is kind
of bond is called a dative covalent bond. [CL3] (2)
8. D Carbon has four electrons in the outermost energy level of its atoms.
is Lewis structure is the only one which correctly shows carbon
atoms with four valence electrons. [CL4] (2)
9. B e element X has six valence electrons therefore it is either a sulfur or
an oxygen atom. e element Y must belong to the halogens because
it has seven valence shell electrons. e only possible choices are
narrowed to choices B and D. However, there are two lone pairs on
atom X, so the shape of the molecule will be angular. [CL4] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 108 2018/12/24 8:26:03 AM
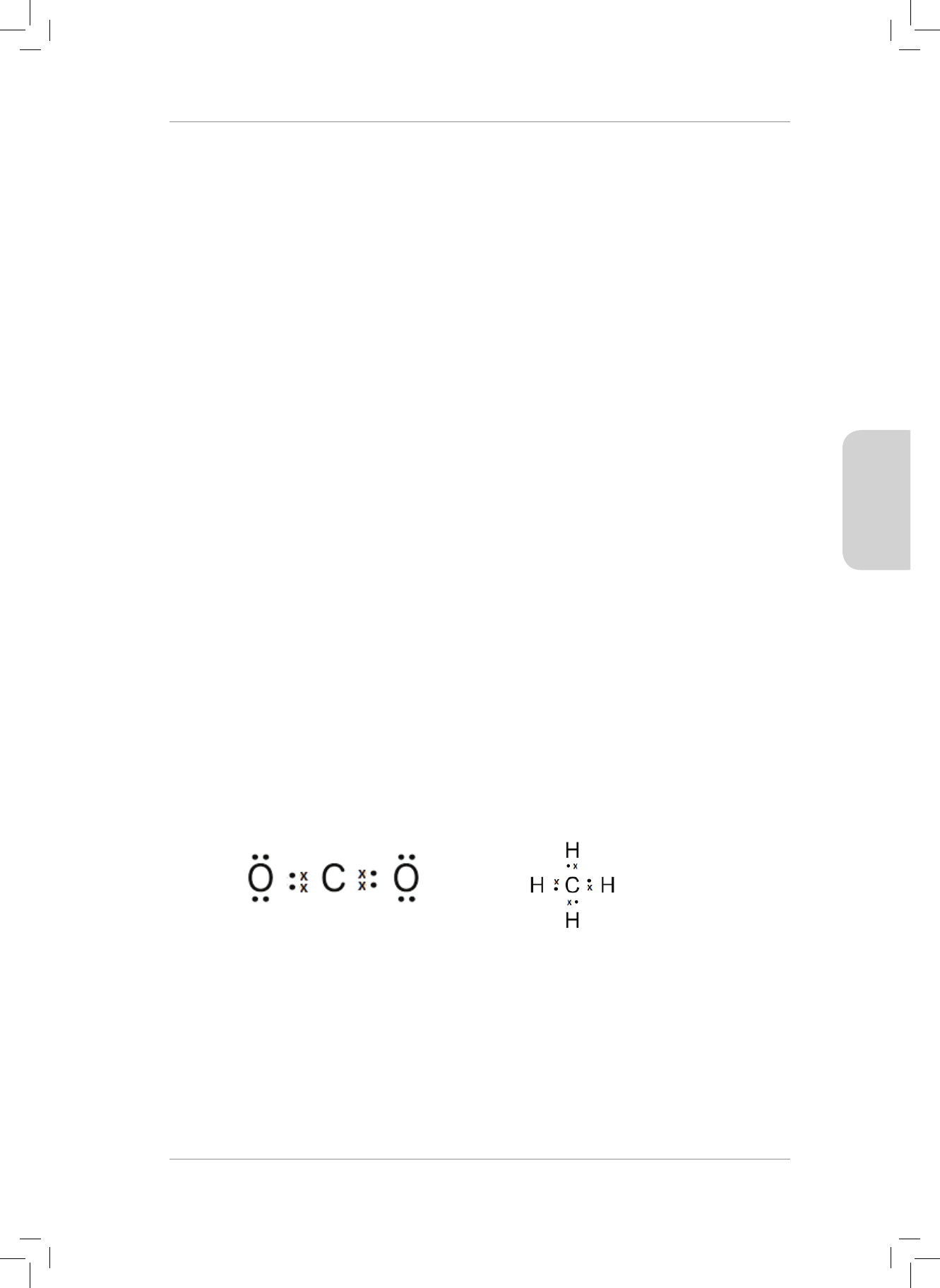
ASSESSMENTS
10. D is molecule has a symmetrical shape (tetrahedral). Even though
it has polar covalent bonds between the atoms, the overall electron
distribution is symmetrical, therefore its molecule is non-polar. [CL3] (2)
LONG QUESTIONS
1.1.1 KI An ionic solid conducts electric current ONLY when molten or dissolve
in water. (1)
1.1.2 C Carbon as diamond is a covalent crystal lattice. (1)
1.1.3 SF
6
e six uorine atoms are arranged symmetrically around the sulfur
atom therefore the overall charge distribution in the molecule is symmetrical – a
non-polar molecule. (1)
1.1.4 H
2
O e oxygen atom has two lone pairs on it in a water molecule. (1)
1.1.5 Hg Mercury is a metal therefore it has delocalised electrons. (1)
1.1.6 H
2
O or PC
3
Shapes are angular (bent) and trigonal pyramidal (each of these
shapes has lone pairs around the central atom, therefore it has a non-ideal shape). (1)
1.1.7 Ne Neon is a noble (inert) gas. Its molecules consist of one atom only
(therefore monatomic). (1)
Because of the choices that learners must make from the list of substances, the
overall cognitive level of this question is Level 3. It requires learners to have a
good understanding and knowledge of molecular geometry and bonding. [CL3]
1.2.1 e bond energy is the amount of energy required to break all the chemical
bonds in one mole of the substance. [CL1] (1)
1.2.2 B [CL2] (1)
e greater the bond energy the shorter the bond length.
1.2.3 e rst bond that forms in a double bond has the same bond energy as a single
bond. It is a non-polar covalent bond between the two carbon atoms. e
second bond that forms has slightly less bond energy as it is a less stable bond.
OR It breaks more easily than the rst single bond. (is is a result of the
orientation of the electron orbitals when the bond is formed.) [CL4] (2)
[11]
2.1.1 a) b) [CL2] (4)
1.1.6 H
2
O or PCℓ
3
Shapes are angular (bent) and trigonal pyramidal (each of these shapes has
lone pairs around the central atom, therefore it has a non-ideal shape. (1)
1.1.7 Ne Neon is a noble (inert) gas. Its molecules consist of one atom only (therefore monatomic).
(1)
Because of the choices that learners must make from the list of substances, the overall
cognitive level of this question is Level 3. It requires learners to have a good understanding and
knowledge of molecular geometry and bonding. [CL3]
1.2.1 The bond energy is the amount of energy required to break all the chemical bonds in one mole
of the substance. [CL1] (1)
1.2.2 B [CL2] (1)
The greater the bond energy the shorter the bond length.
1.2.3 The first bond that forms in a double bond has the same bond energy as a single bond. It is a
non-polar covalent bond between the two carbon atoms. The second bond that forms has
slightly less bond energy as it is a less stable bond. OR It breaks more easily than the first
single bond. (This is a result of the orientation of the electron orbitals when the bond is formed.)
[CL4] (2)
[11]
2.1.1 a) b) [CL2} (4)
Correct number of electrons around each atom.
Correct positioning of chemical bonds.
(2 marks for each Lewis diagram)
2.1.2 The electronegativity is a measure of the tendency of an atom in a molecule to attract the
bonding electrons. [CL1] (2)
2.1.3 S: EN = 2,5 O: EN = 3,5
∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond {CL2} (2)
2.1.4 The methane molecule has a tetrahedral shape. Its polar covalent bonds are arranged
symmetrically within the molecule therefore the overall electron distribution within the
molecule is symmetrical – and the molecule is non-polar.
Lewis diagram with dipoles shown
[CL3] (4)
2.1.5 a) NH
3
b) CH
4
1.1.6 H
2
O or PCℓ
3
Shapes are angular (bent) and trigonal pyramidal (each of these shapes has
lone pairs around the central atom, therefore it has a non-ideal shape. (1)
1.1.7 Ne Neon is a noble (inert) gas. Its molecules consist of one atom only (therefore monatomic).
(1)
Because of the choices that learners must make from the list of substances, the overall
cognitive level of this question is Level 3. It requires learners to have a good understanding and
knowledge of molecular geometry and bonding. [CL3]
1.2.1 The bond energy is the amount of energy required to break all the chemical bonds in one mole
of the substance. [CL1] (1)
1.2.2 B [CL2] (1)
The greater the bond energy the shorter the bond length.
1.2.3 The first bond that forms in a double bond has the same bond energy as a single bond. It is a
non-polar covalent bond between the two carbon atoms. The second bond that forms has
slightly less bond energy as it is a less stable bond. OR It breaks more easily than the first
single bond. (This is a result of the orientation of the electron orbitals when the bond is formed.)
[CL4] (2)
[11]
2.1.1 a) b) [CL2} (4)
Correct number of electrons around each atom.
Correct positioning of chemical bonds.
(2 marks for each Lewis diagram)
2.1.2 The electronegativity is a measure of the tendency of an atom in a molecule to attract the
bonding electrons. [CL1] (2)
2.1.3 S: EN = 2,5 O: EN = 3,5
∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond {CL2} (2)
2.1.4 The methane molecule has a tetrahedral shape. Its polar covalent bonds are arranged
symmetrically within the molecule therefore the overall electron distribution within the
molecule is symmetrical – and the molecule is non-polar.
Lewis diagram with dipoles shown
[CL3] (4)
2.1.5 a) NH
3
b) CH
4
Correct number of electrons around each atom.
Correct positioning of chemical bonds.
(2 marks for each Lewis diagram)
2.1.2 e electronegativity is a measure of the tendency of an atom in a molecule to
attract the bonding electrons. [CL1] (2)
2.1.3 S: EN = 2,5 O: EN = 3,5
∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond [CL2] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 109 2018/12/24 8:26:04 AM
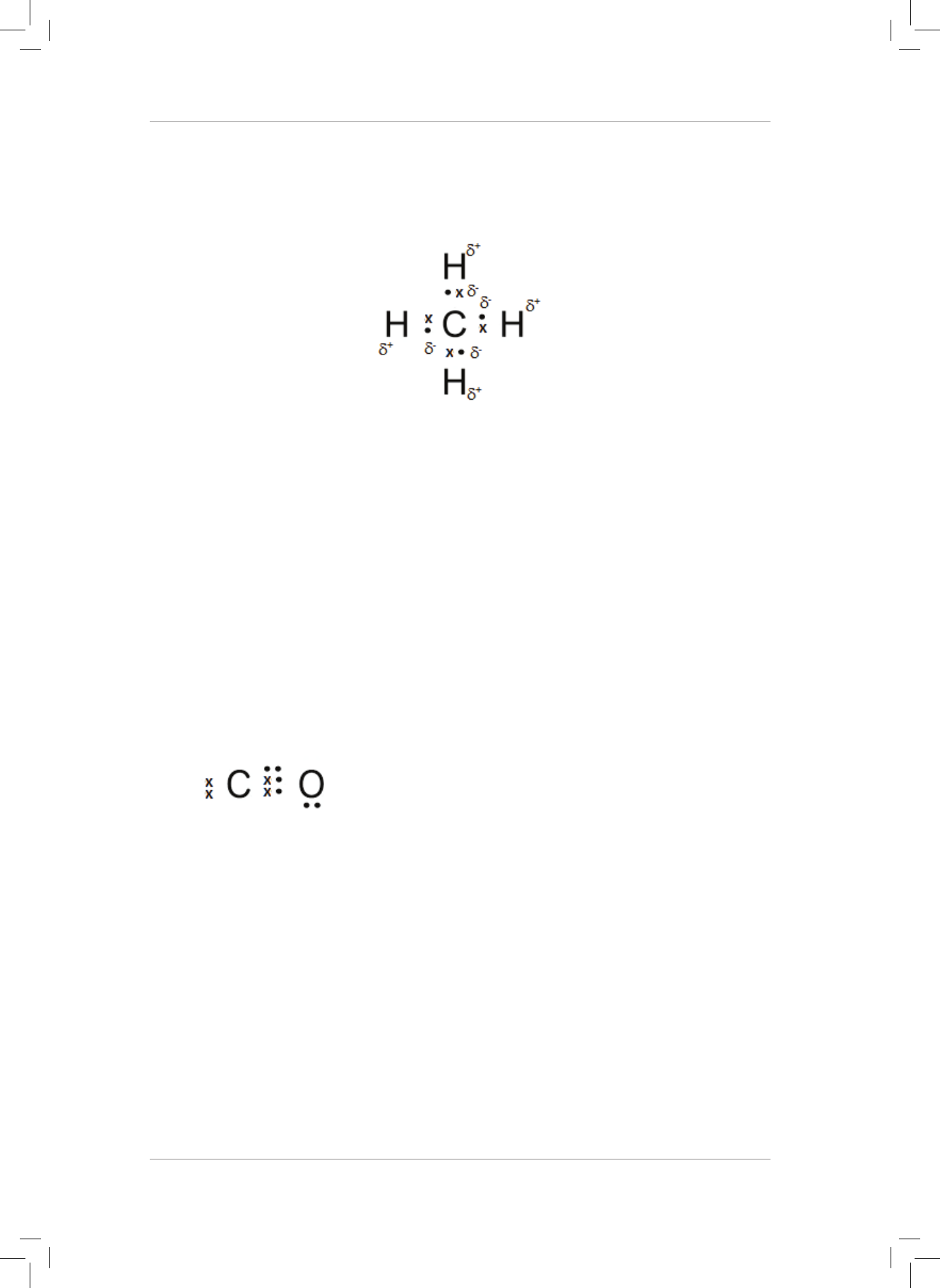
RESOURCE PACK
2.1.4 e methane molecule has a tetrahedral shape. Its polar covalent bonds are
arranged symmetrically within the molecule therefore the overall electron
distribution within the molecule is symmetrical – and the molecule is non-polar.
Lewis diagram with dipoles shown
1.1.6 H
2
O or PCℓ
3
Shapes are angular (bent) and trigonal pyramidal (each of these shapes has
lone pairs around the central atom, therefore it has a non-ideal shape. (1)
1.1.7 Ne Neon is a noble (inert) gas. Its molecules consist of one atom only (therefore monatomic).
(1)
Because of the choices that learners must make from the list of substances, the overall
cognitive level of this question is Level 3. It requires learners to have a good understanding and
knowledge of molecular geometry and bonding. [CL3]
1.2.1 The bond energy is the amount of energy required to break all the chemical bonds in one mole
of the substance. [CL1] (1)
1.2.2 B [CL2] (1)
The greater the bond energy the shorter the bond length.
1.2.3 The first bond that forms in a double bond has the same bond energy as a single bond. It is a
non-polar covalent bond between the two carbon atoms. The second bond that forms has
slightly less bond energy as it is a less stable bond. OR It breaks more easily than the first
single bond. (This is a result of the orientation of the electron orbitals when the bond is formed.)
[CL4] (2)
[11]
2.1.1 a) b) [CL2} (4)
Correct number of electrons around each atom.
Correct positioning of chemical bonds.
(2 marks for each Lewis diagram)
2.1.2 The electronegativity is a measure of the tendency of an atom in a molecule to attract the
bonding electrons. [CL1] (2)
2.1.3 S: EN = 2,5 O: EN = 3,5
∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond {CL2} (2)
2.1.4 The methane molecule has a tetrahedral shape. Its polar covalent bonds are arranged
symmetrically within the molecule therefore the overall electron distribution within the
molecule is symmetrical – and the molecule is non-polar.
Lewis diagram with dipoles shown
[CL3] (4)
2.1.5 a) NH
3
b) CH
4
[CL3] (4)
2.1.5 a) NH
3
b) CH
4
c) CO
2
or CO or NO [CL3] (3)
2.2.1 e bond length is the average distance between the nuclei of two bonded atoms.
[CL1] (2)
2.2.2 a) 120 pm [CL3] (1)
b) 800 kJmol
-1
[CL2] (2)
c) Bond energy: the amount of energy per mole of the substance required to
break the double bond between the carbon and oxygen atoms. [CL2] (1)
2.2.3 LONGER THAN e sulfur atom is larger than the carbon atom therefore
the bond length will be longer (the attraction between the two atoms will be
less). [CL4] (2)
2.2.4 At point D there is very little (or no) force between the two atoms because the
atoms are fairly far apart from one another. [CL3] (2)
[25]
3.1.1
c) CO
2
or CO or NO [CL3] (3)
2.2.1 The bond length is the average distance between the nuclei of two bonded atoms.
[CL1] (2)
2.2.2 a) 120 pm [CL3] (1)
b) 800 kJ⋅mol
-1
[CL2] (2)
c) Bond energy: the amount of energy per mole of the substance required to break the
double bond between the carbon and oxygen atoms. [CL2] (1)
2.2.3 LONGER THAN The sulfur atom is larger than the carbon atom therefore the bond length
will be longer (the attraction between the two atoms will be less). [CL4] (2)
2.2.4 At point D there is very little (or no) force between the two atoms because the atoms are fairly
far apart from one another. (OR The forces of attraction and repulsion are balanced.)
[CL3] (2)
[25]
3.1.1
Dative covalent bond occurs when one atom contributes both electrons (from a lone pair of
electrons) to the bond. In this case oxygen donates the electrons to the dative covalent
bond. [CL4] (4)
3.1.2 ∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond
The molecule is linear therefore it is a dipole (polar molecule). {CL2} (2)
3.1.3 Oxygen is more electronegative than carbon therefore the shared pairs of electrons will be more
strongly attracted to oxygen making it slight negative (δ
-
). Carbon will be δ
+
.
[CL3] (2)
3.2.1 The CO
2
molecule is linear. It’s shape is ideal since there are no lone pairs in the molecule.
The SO
2
molecule is angular (bent) since there are two lone pairs on the central atom (S).
[CL4] (4)
3.2.2 The CO
2
molecule is non-polar because although the bonds are polar covalent, they are
arranged symmetrically in the molecule, so the overall electron distribution is also
symmetrical. The SO
2
molecule has a bent structure since the lone pairs repel each other
strongly. Oxygen is more electronegative than sulfur, therefore the oxygen atoms will be
slightly negative (δ
-
) and the sulfur atom will be slightly positive (δ
+
) (a polar molecule).
[CL3] (4)
[16]
4.1.1 a)
Correct sodium ion
Two sodium ions
Correct sulfide ion [CL3] (3)
Dative covalent bond occurs when one atom contributes both electrons (from a
lone pair of electrons) to the bond. In this case oxygen donates the electrons
to the dative covalent bond. [CL4] (4)
3.1.2 ∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond
e molecule is linear therefore it is a dipole (polar molecule). [CL2] (2)
3.1.3 Oxygen is more electronegative than carbon therefore the shared pairs of
electrons will be more strongly attracted to oxygen making it slight negative
(δ
-
). Carbon will be δ
+
. [CL3] (2)
3.2.1 e CO
2
molecule is linear. It’s shape is ideal since there are no lone pairs in
the molecule.
e SO
2
molecule is angular (bent) since there are two lone pairs on the
central atom (S). [CL4] (4)
Gr11_Physical_Science_Term1_Resource_Book.indb 110 2018/12/24 8:26:05 AM
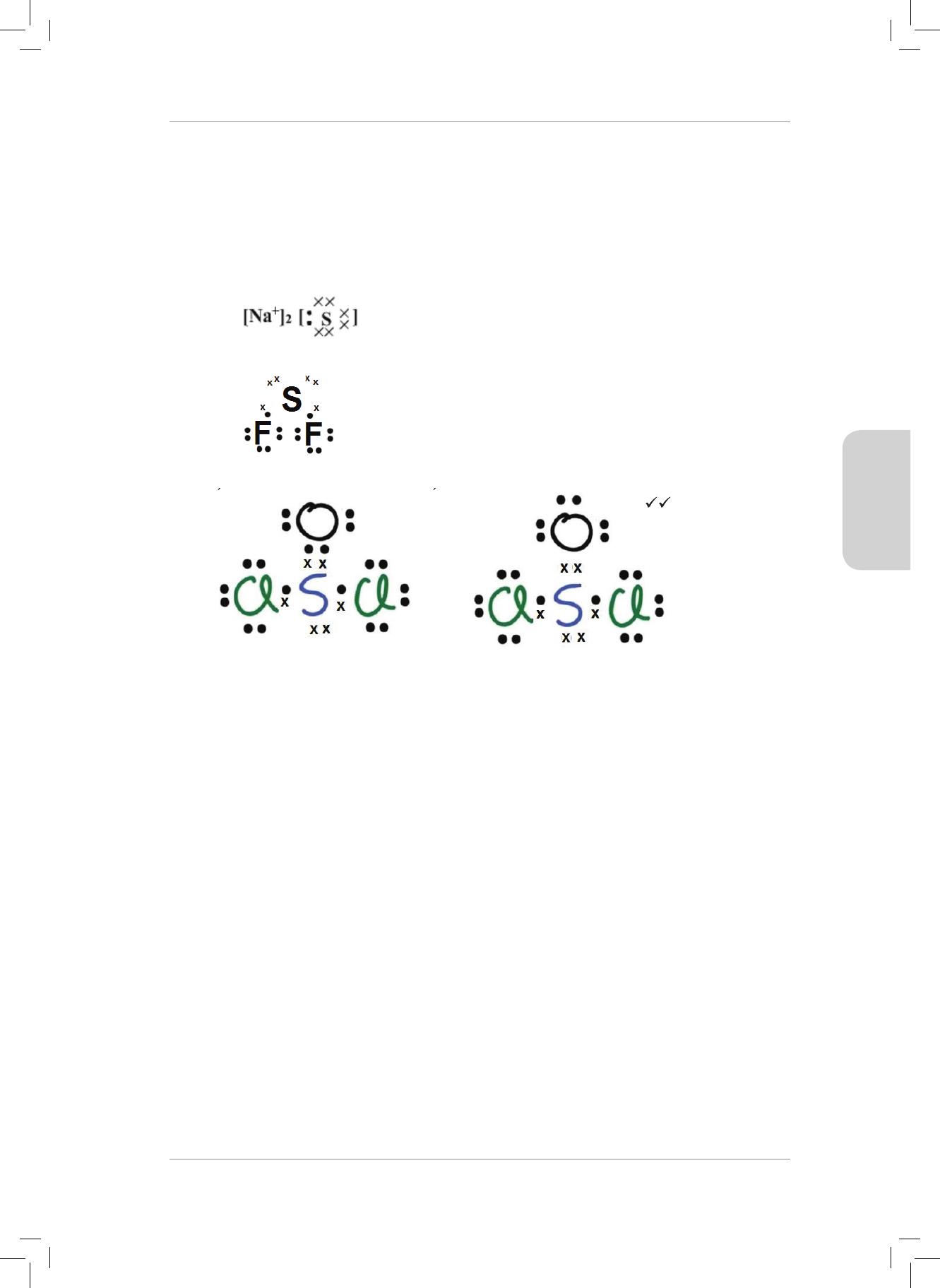
ASSESSMENTS
3.2.2 e CO
2
molecule is non-polar because although the bonds are polar covalent,
they are arranged symmetrically in the molecule, so the overall electron
distribution is also symmetrical. e SO
2
molecule has a bent structure since
the lone pairs repel each other strongly. Oxygen is more electronegative than
sulfur, therefore the oxygen atoms will be slightly negative (δ
-
) and the sulfur
atom will be slightly positive (δ
+
) (a polar molecule). [CL3] (4)
4.1.1 a)
Correct sodium ion
c) CO
2
or CO or NO [CL3] (3)
2.2.1 The bond length is the average distance between the nuclei of two bonded atoms.
[CL1] (2)
2.2.2 a) 120 pm [CL3] (1)
b) 800 kJ⋅mol
-1
[CL2] (2)
c) Bond energy: the amount of energy per mole of the substance required to break the
double bond between the carbon and oxygen atoms. [CL2] (1)
2.2.3 LONGER THAN The sulfur atom is larger than the carbon atom therefore the bond length
will be longer (the attraction between the two atoms will be less). [CL4] (2)
2.2.4 At point D there is very little (or no) force between the two atoms because the atoms are fairly
far apart from one another. (OR The forces of attraction and repulsion are balanced.)
[CL3] (2)
[25]
3.1.1
Dative covalent bond occurs when one atom contributes both electrons (from a lone pair of
electrons) to the bond. In this case oxygen donates the electrons to the dative covalent
bond. [CL4] (4)
3.1.2 ∆ electronegativity = 3,5 – 2,5 = 1,0
Polar covalent bond
The molecule is linear therefore it is a dipole (polar molecule). {CL2} (2)
3.1.3 Oxygen is more electronegative than carbon therefore the shared pairs of electrons will be more
strongly attracted to oxygen making it slight negative (δ
-
). Carbon will be δ
+
.
[CL3] (2)
3.2.1 The CO
2
molecule is linear. It’s shape is ideal since there are no lone pairs in the molecule.
The SO
2
molecule is angular (bent) since there are two lone pairs on the central atom (S).
[CL4] (4)
3.2.2 The CO
2
molecule is non-polar because although the bonds are polar covalent, they are
arranged symmetrically in the molecule, so the overall electron distribution is also
symmetrical. The SO
2
molecule has a bent structure since the lone pairs repel each other
strongly. Oxygen is more electronegative than sulfur, therefore the oxygen atoms will be
slightly negative (δ
-
) and the sulfur atom will be slightly positive (δ
+
) (a polar molecule).
[CL3] (4)
[16]
4.1.1 a)
Correct sodium ion
Two sodium ions
Correct sulfide ion [CL3] (3)
Two sodium ions
Correct sul de ion [CL3] (3)
b)
Correct number of electrons sulfur atom
Correct uoride ions
b)
Correct number of electrons sulfur atom
Correct fluoride ions [CL3] (2)
4.1.2 a) b)
One mark for each
Sulfur is the central atom with its six valence electrons. Oxygen also has six valence electrons
and chlorine has seven valence electrons. In the first molecule sulfur and oxygen form a
double bond between them; in the second molecule there is a dative covalent bond between
sulfur and oxygen. [CL4] (5)
4.1.3 a) SF
2
Angular Two lone pairs on sulfur atom repel each other causing the shape
to be bent (angular). [CL3] (2)
b) BeCℓ
2
Linear An ideal shape as there are no lone pairs on Be.
[CL2] (2)
4.2.1 a) Electronegativity: a measure of the tendency of an atom in a molecule to attract the
bonding electrons. [CL1] (2)
b) Bond polarity: A non-polar covalent bond is one in which the electron density is shared
equally between the bonding atoms, and a polar covalent bon is one in which the electron
density is shared unequally between the bonding atoms.
[CL1] (2)
4.2.2 a) SF
2
Polar covalent bonds
∆ electronegativity (method) = 4,0 – 2,5 = 1,5 [CL2] (3)
b) H
2
S Polar covalent bonds
∆ electronegativity = 2,5 – 2,1 = 0,4 [CL2] (2)
[21]
5.1.1 electrons [CL2] (1)
5.1.2 polar covalent bonds [CL2] (1)
5.2.1 H
+
ion (or H
3
O
+
ion or hydrogen ion or hydronium ion) [CL3] (1)
5.2.2 HI
It requires the lowest amount of energy to break the bond between H and I therefore it will
react most readily with water to release H
=
ions. [CL4] (3)
[6]
6.1.1 7 [CL2] (1)
[CL3] (2)
4.1.2 a) b)
One mark
for each
4.1.2 a) b)
One mark
Sulfur is the central atom with its six valence electrons. Oxygen also has
six valence electrons and chlorine has seven valence electrons. In the rst
molecule sulfur and oxygen form a double bond between them; in the second
molecule there is a dative covalent bond between sulfur and oxygen. [CL4] (5)
4.1.3 a) SF
2
Angular Two lone pairs on sulfur atom repel each other
causing the shape to be bent (angular). [CL3] (2)
b) BeC
2
Linear An ideal shape as there are no lone pairs on Be.
[CL2] (2)
4.2.1 a) Electronegativity: a measure of the tendency of an atom in a molecule to
attract the bonding electrons. [CL1] (2)
b) Bond polarity: A non-polar covalent bond is one in which the electron
density is shared equally between the bonding atoms, and a polar covalent
bond is one in which the electron density is shared unequally between the
bonding atoms. [CL1] (2)
4.2.2 a) SF
2
Polar covalent bonds
∆ electronegativity (method) = 4,0 – 2,5 = 1,5 [CL2] (3)
b) H
2
S Polar covalent bonds
∆ electronegativity = 2,5 – 2,1 = 0,4 [CL2] (2)
[21]
Gr11_Physical_Science_Term1_Resource_Book.indb 111 2018/12/24 8:26:07 AM

RESOURCE PACK
5.1.1 electrons [CL2] (1)
5.1.2 polar covalent bonds [CL2] (1)
5.2.1 H
+
ion (or H
3
O
+
ion or hydrogen ion or hydronium ion) [CL3] (1)
5.2.2 HI
It requires the lowest amount of energy to break the bond between H and I
therefore it will react most readily with water to release H
=
ions. [CL4] (3)
[6]
6.1.1 7 [CL2] (1)
6.1.2 4 [CL2] (1)
6.2 A covalent bond is the sharing of electrons to form a chemical bond between
two atoms. [CL!] (2)
6.3.1
correct number of valence electrons in each atom
6.1.2 4 [CL2] (1)
6.2 A covalent bond is the sharing of electrons to form a chemical bond between two atoms.
[CL!] (2)
6.3.1
correct number of valence electrons in each atom
covalent bond shown clearly [CL2] (2)
6.3.2 correct number of valence electrons in each atom
covalent bonds shown clearly [CL2] (2)
6.4.1 covalent bond (non-polar) [CL2] (1)
6.4.2 polar covalent bond [CL2] (1)
6.5.1 trigonal planar [CL2] (2)
6.5.2 tetrahedral [CL2] (2)
6.5.3 linear [CL2] (2)
6.6.1 The shorter the bond length the higher the bond energy. [CL3] (2)
6.6.2 C C can break to form B losing 839 – 614 = 225 kJ⋅mol
-1
which is less than the energy
required to break a single covalent bond between the carbon atoms. {CL4] (3)
[20]
covalent bond shown clearly [CL2] (2)
6.3.2
correct number of valence electrons in each atom
6.1.2 4 [CL2] (1)
6.2 A covalent bond is the sharing of electrons to form a chemical bond between two atoms.
[CL!] (2)
6.3.1
correct number of valence electrons in each atom
covalent bond shown clearly [CL2] (2)
6.3.2 correct number of valence electrons in each atom
covalent bonds shown clearly [CL2] (2)
6.4.1 covalent bond (non-polar) [CL2] (1)
6.4.2 polar covalent bond [CL2] (1)
6.5.1 trigonal planar [CL2] (2)
6.5.2 tetrahedral [CL2] (2)
6.5.3 linear [CL2] (2)
6.6.1 The shorter the bond length the higher the bond energy. [CL3] (2)
6.6.2 C C can break to form B losing 839 – 614 = 225 kJ⋅mol
-1
which is less than the energy
required to break a single covalent bond between the carbon atoms. {CL4] (3)
[20]
covalent bonds shown clearly [CL2] (2)
6.4.1 covalent bond (non-polar) [CL2] (1)
6.4.2 polar covalent bond [CL2] (1)
6.5.1 trigonal planar [CL2] (2)
6.5.2 tetrahedral [CL2] (2)
6.5.3 linear [CL2] (2)
6.6.1 e shorter the bond length the higher the bond energy. [CL3] (2)
6.6.2 C C can break to form B losing 839 – 614 = 225 kJmol
-1
which is less than
the energy required to break a single covalent bond between the carbon atoms.
[CL4] (3)
[20]
Gr11_Physical_Science_Term1_Resource_Book.indb 112 2018/12/24 8:26:09 AM
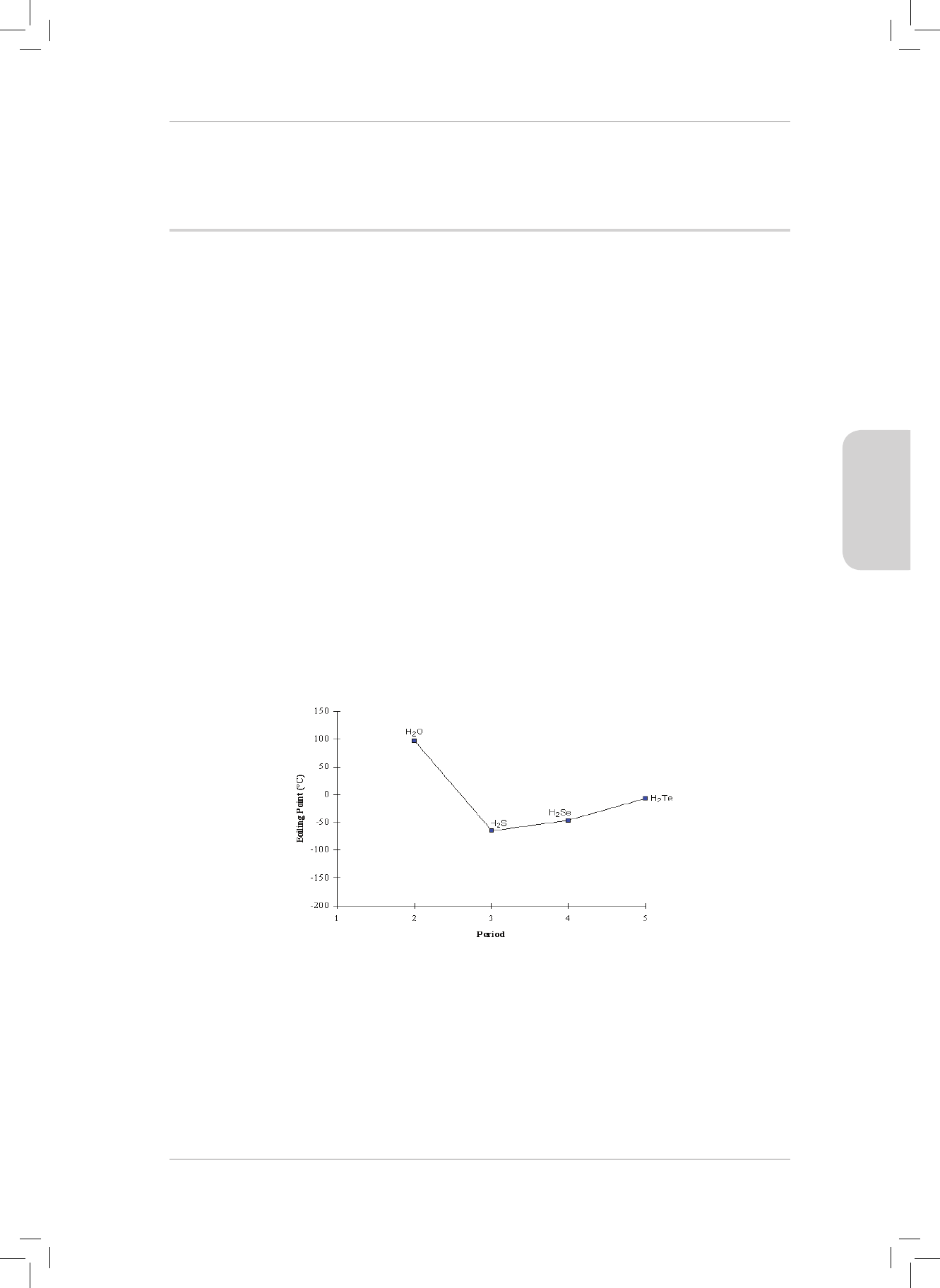
ASSESSMENTS
Topic 4: Intermolecular Forces
QUESTIONS
MULITPLE-CHOICE QUESTIONS
1. Between which particles can hydrogen bonds occur?
A Small molecules that contain hydrogen.
B Molecules in which hydrogen is bonded to small atoms with high
electronegativity.
C Small atoms with very high electronegativity.
D Molecules in which hydrogen is bonded to small atoms with low
electronegativity. (2)
2. e gecko is a small lizard that can climb up a smooth glass window. e gecko
has millions of microscopic hairs on its toes and each hair has thousands of pads
on its tip. e result is that the molecules in the pads are extremely close to the
glass surface on which the gecko is climbing.
What is the attraction between the gecko’s toe pads and the glass surface?
A dative covalent bonds
B covalent bonds
C ionic bonds
D van der Waals forces (2)
Questions 3. and 4. refer to the graph below.
GRADE 11: TERM 1 ASSESSMENT: QUESTIONS TOPIC 4
MULITPLE-CHOICE QUESTIONS
1.1 Between which particles can hydrogen bonds occur?
A Small molecules that contain hydrogen.
B Molecules in which hydrogen is bonded to small atoms with high
electronegativity.
C Small atoms with very high electronegativity.
D Molecules in which hydrogen is bonded to small atoms with low
electronegativity. (2)
1.2 The gecko is a small lizard that can climb up a smooth glass window. The gecko has
millions of microscopic hairs on its toes and each hair has thousands of pads on its
tip. The result is that the molecules in the pads are extremely close to the glass
surface on which the gecko is climbing.
What is the attraction between the gecko’s toe pads and the glass surface?
A dative covalent bonds
B covalent bonds
C ionic bonds
D van der Waals forces (2)
Questions 1.3 and 1.4 refer to the graph below.
1.3 Water has a higher boiling point than expected because it…
A is a liquid.
B has strong hydrogen bonding intermolecular forces.
C is a polar covalent molecule.
3. Water has a higher boiling point than expected because it…
A is a liquid.
B has strong hydrogen bonding intermolecular forces.
C is a polar covalent molecule.
D has small polar molecules.
Gr11_Physical_Science_Term1_Resource_Book.indb 113 2018/12/24 8:26:09 AM

RESOURCE PACK
4. e tendency for boiling point to increase from H
2
S to H
2
Te is due to…
A stronger London intermolecular forces.
B stronger polar covalent bonds.
C increasing molecular polarity.
D stronger dipole-dipole intermolecular forces.
5. Which one of the following combinations is correct?
Compound Strongest Intermolecular force
A CH
3
B
C CH
3
OH
D
4
(2)
6. e fact that noble gases liquefy can be given as evidence for the existence of …
A covalent bonds.
B dipole-dipole forces.
C hydrogen bonds.
D London forces. (2)
7. Crude oil consists of a mixture of dierent compounds called hydrocarbons. e
molecular formula of some of these compounds is given below. Which one of
these compounds will have the highest boiling point?
A C
4
H
10
B C
6
H
14
C C
8
H
18
D C
10
H
22
(2)
Gr11_Physical_Science_Term1_Resource_Book.indb 114 2018/12/24 8:26:09 AM
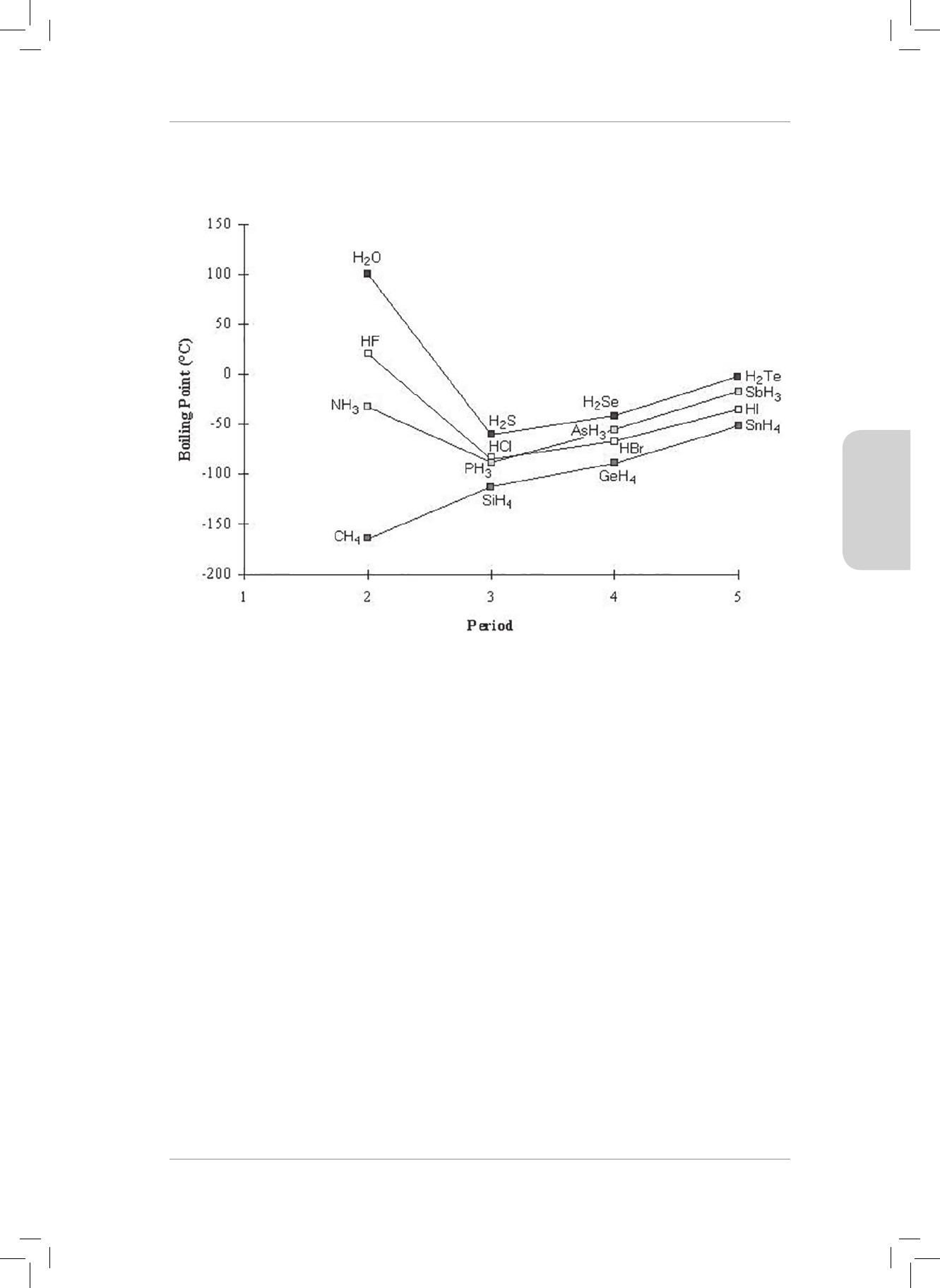
ASSESSMENTS
8. e graph shown below gives the boiling points of the hydrides of group 14, 15,
16 and 17 elements.
Water, hydrogen uoride and ammonia have higher boiling points than expected,
which suggests that these substances have stronger intermolecular forces between
their molecules.
Which of the following does not contribute to the unexpectedly high boiling
points of these three substances?
Nitrogen, oxygen and uorine ……
A have small atoms.
B are non-metals.
C have high electronegativities.
D are bonded to hydrogen atoms. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 115 2018/12/24 8:26:10 AM

RESOURCE PACK
9. Hydrogen chloride occurs naturally as a gas at room temperature. When the
temperature is reduced, hydrogen chloride liquees and eventually solidies.
Which of the following pairs correctly names the bonds and the intermolecular
forces found in hydrogen chloride?
Bond Intermolecular force
A
C
(2)
10. In which one of the following compounds will hydrogen bonds be present? (All
these compounds are in the liquid phase).
A HC
B HF
C H
2
D H
2
S (2)
LONG QUESTIONS
1. Water exists as a liquid at room temperature, but methane exists as a gas at this
temperature. e molar mass of water is 18 gmol
-1
and that of methane is
16gmol
-1
. Explain, by discussing their intra- and intermolecular forces, why
these two substances of similar molar mass exist in dierent phases at room
temperature. [6]
2. H
2
O and CC
4
are both liquids that also act as solvents for other substances. CC
4
has been used in the dry-cleaning business, but because it is toxic, it is no longer
used for this commercially.
e table below gives information about these two substances.
Substance Viscosity (kgm
-1
s) Surface tension (Jm
-2
)
H
2
O
1 × 10
-3
× 10
-2
4
× 10
-4
× 10
-2
2.1 Determine the types of bonds in each of these substances. Justify your answer. (3)
2.2 Draw Lewis dot diagrams for:
a) H
2
O
b) CC
4
(4)
2.3 Identify the specic type of intermolecular forces in each compound. (2)
2.4 Explain the dierences in viscosity and the surface tension of these two
substances as shown in the data in the table above. (5)
2.5 KC and I
2
are both solids at room temperature. Which of these substances
will dissolve most readily in CC
4
? Explain briey. (3)
[17]
Gr11_Physical_Science_Term1_Resource_Book.indb 116 2018/12/24 8:26:10 AM

ASSESSMENTS
3.1 Consider the following information for the compounds CF
4
and NH
3
at
standard pressure.
Compound Melting Point (°C) Boiling Point (°C) Solubility in Water
at 20 °C
4
− −
3
− −
3.1.1 What evidence is there in the table that suggests that NH
3
molecules
experience stronger intermolecular forces than CF
4
? (1)
3.1.2 Explain fully why CF
4
is insoluble in water but NH
3
is soluble. (5)
3.1.3 Ammonia dissolves in water to form the ammonium anion.
(a) Write down the ionic equation for the reaction. (1)
(b) Explain the formation of a dative covalent bond in ammonia. (2)
3.2 e graph below shows the relationship between the boiling points of
the hydrides of group VI elements, namely H
2
W, H
2
X, H
2
Y and H
2
Z, and
molecular mass.
3.2. The graph below shows the relationship between the boiling points of the hydrides of
group VI elements, namely H
2
W, H
2
X, H
2
Y and H
2
Z, and molecular mass.
3.2.1. What unexpected result do you observe on the graph?
(2)
3.2.2. Identify H
2
W.
(2)
3.2.3. Explain the phenomenon referred to in question 3.2.1.
(4)
[17]
4. The equation for the combustion reaction of ethanol is given below.
C
2
H
5
OH() + 3 O
2
(g) 2 CO
2
(g) + 3 H
2
O() ΔH < 0
4.4.1 Draw the Lewis electron dot diagram for the ethanol molecule (CH
3
CH
2
OH).
Use dots and
crosses or different colours to show which electrons come from which atoms.
(3)
4.4.2 Name and define the specific type of intramolecular bond that forms between
the C atom and the O atom in the ethanol molecule. (3)
4.4.3 Name/state the molecular geometry (shape) around…
(a) each C atom in the ethanol molecule. (1)
(b) the O atom in the ethanol molecule. (1)
4.4.4 Explain clearly why ethanol is a versatile (very useful) solvent. (4)
[12]
5. The table below shows the boiling points of four alcohols.
3.2.1 What unexpected result do you observe on the graph? (2)
3.2.2 Identify H
2
W. (2)
3.2.3 Explain the phenomenon referred to in question 3.2.1. (4)
[17]
Gr11_Physical_Science_Term1_Resource_Book.indb 117 2018/12/24 8:26:10 AM
RESOURCE PACK
4. e equation for the combustion reaction of ethanol is given below.
C
2
H
5
OH() + 3 O
2
(g) 2 CO
2
(g) + 3 H
2
O() H < 0
4.1 Draw the Lewis electron dot diagram for the ethanol molecule
(CH
3
CH
2
OH). Use dots and crosses or dierent colours to show which
electrons come from which atoms. (3)
4.2 Name and dene the specic type of intramolecular bond that forms
between the C atom and the O atom in the ethanol molecule. (3)
4.3 Name/state the molecular geometry (shape) around…
(a) each C atom in the ethanol molecule. (1)
(b) the O atom in the ethanol molecule. (1)
4.4 Explain clearly why ethanol is a versatile (very useful) solvent. (4)
[12]
5. e table below shows the boiling points of four alcohols.
Alcohol Molecular formula Boiling point (°C)
CH
3
OH 65
C
2
H
5
OH 79
C
3
H
7
OH 97
C
4
H
9
OH 117
5.1 Draw a graph of the boiling points (on the y-axis) against the number of
carbon atoms in the molecule (on the x-axis). Start with the temperatures
from 60°C. Draw the line of best t for the data. (5)
5.2 Describe the trend in the boiling points of the alcohols. (2)
5.3 Name the strongest intermolecular forces in these substances. (1)
5.4 Explain the trend that this data shows about boiling points of alcohols, with
reference to the intermolecular forces. (2)
5.5 Methanol (CH
3
OH) has a boiling point of 65°C whereas ethane (C
2
H
6
) boils
at – 164°C. Explain why these two substances have such wide dierences in
their boiling points even though they have the same molecular mass. (4)
[14]
6. e boiling points of four compounds of hydrogen at standard pressure are given
in the table below.
Formula Boiling point
(°C)
CH
4
-164
NH
3
−33
H
2
O 100
SiH
4
−112
6.1 Dene the term ‘boiling point’. (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 118 2018/12/24 8:26:10 AM

ASSESSMENTS
6.2 Fully explain the dierence in boiling points between CH
4
and
6.2.1 NH
3
. (3)
6.2.2 SiH
4
. (3)
6.3 Explain why the boiling points of NH
3
and H
2
O dier by referring to
electronegativity, the molecular shapes and the intermolecular forces in
these substances. (4)
6.4 Select a substance from the table above which has
6.4.1 polar molecules. (1)
6.4.2 non-polar molecules. (1)
[14]
7. e properties of water play an important role for survival on Earth.
7.1 Explain why the density of water decreases when it freezes. (3)
7.2 Give ONE reason why this decrease in the density of water is important for
life on Earth. (2)
7.3 Briey explain, by referring to the unique property of water involved, how
water regulates temperatures on Earth. (2)
7.4 Which ONE of intermolecular forces or intramolecular forces is stronger?
Explain your answer using water as an example. (3)
7.5 Water evaporates slower than eucalyptus oil. Explain this statement in terms
of intermolecular forces. (3)
[13]
Gr11_Physical_Science_Term1_Resource_Book.indb 119 2018/12/24 8:26:11 AM
RESOURCE PACK
MARKING GUIDELINES
MULTIPLE CHOICE
1. B It is the small atom with high electronegativity that is bonded to a
hydrogen atom that allows hydrogen bonds to form between molecules
of a substance. [CL2] (2)
2. D It is the force between the molecules of glass and the molecules in the
pads of gecko’s toes that allows it climb the smooth glass surface. e
other choices here concern intramolecular forces (chemical bonds). e
gecko does not bond permanently with the glass! [CL3] (2)
3. B e other substances do not have hydrogen bonds between their
molecules. It must therefore be the strong hydrogen bonds between
water molecules that accounts for its high boiling point. [CL2] (2)
4. D e molecules of H
2
S, H
2
Se and H
2
Te are polar, therefore the
intermolecular forces between them are dipole-dipole forces. e forces
become stronger as the size of the molecules increases. [CL2] (2)
5. D CH
3
F does not have hydrogen bonding because the uorine atom
is attached to the carbon atom (not to a hydrogen atom). HC has
dipole-dipole forces (not hydrogen bonds). CH
3
OH has dipole-dipole
forces, but its strongest intermolecular forces are hydrogen bonds. e
only correct answer is therefore CC
4
which does have London forces
between its molecules. [CL3] (2)
6. D e noble gases are non-polar substances. e fact that they liquefy
gives evidence that forces exist between their molecules in the liquid
phase. ese forces are London forces. [CL2] (2)
7. D ese compounds are all the same type of substance; therefore,
they will have the same type of intermolecular forces between their
molecules. e intermolecular forces will increase in strength as the
size of the molecules increases because there will be more points of
contact available for the forces to hold the molecules closer together.
[CL2] (2)
8. B It is the small atom with high electronegativity that is bonded to a
hydrogen atom that allows hydrogen bonds to form between molecules of
a substance. erefore, the fact that these elements are non-metals does not
contribute directly to the hydrogen bonding. [CL 4] (2)
Gr11_Physical_Science_Term1_Resource_Book.indb 120 2018/12/24 8:26:11 AM
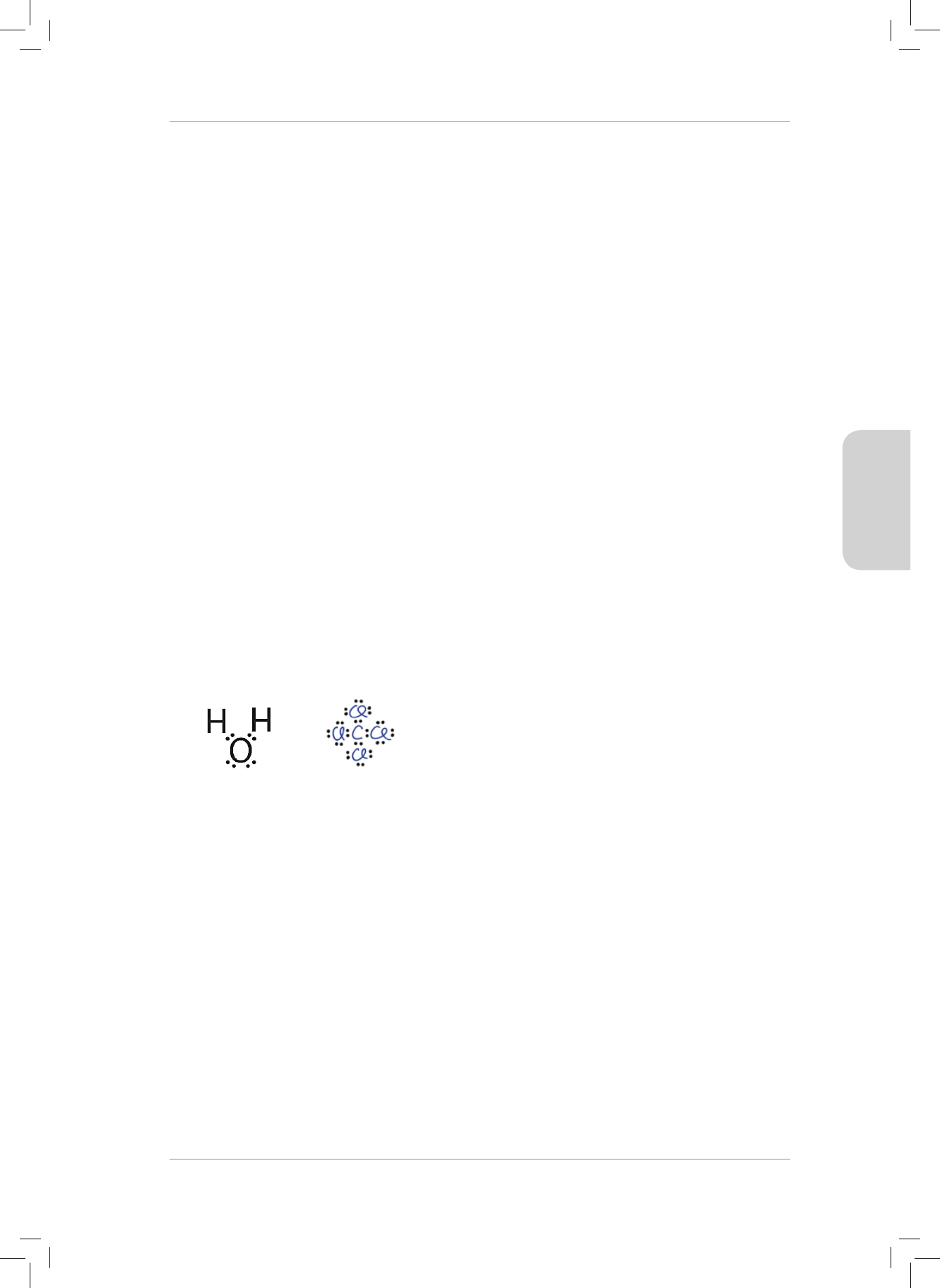
ASSESSMENTS
9. D Hydrogen chloride has one polar covalent bond between its atoms. It
has dipole-dipole intermolecular forces between its molecules. [CL 3] (2)
10. B F is a small atom with high electronegativity bonded to H. It is these
types of molecules that can form hydrogen bonds between them. [CL2] (2)
LONG QUESTIONS
1. Both methane and water have polar covalent bonds between their atoms.
∆ electronegativity C-H = 2,5 – 2,1 = 0,4
∆ electronegativity H-0 = 3,5 – 2,1 = 1,4
(for calculating ∆ electronegativity correctly)
e bond between oxygen and hydrogen is more polar than that between carbon
and hydrogen.
Water has an angular structure, whereas methane is tetrahedral. e electron
distribution in the water molecule is asymmetrical, but it is symmetrical in the
methane molecule.
Water molecules are polar; methane molecules are non-polar.
e intermolecular forces between water molecules are hydrogen bond (dipole-
dipole) forces which are much stronger than the London forces (induced
dipole-induced dipole forces) that exist between methane molecules, therefore
water is a liquid and methane is a gas at room temperature. [CL 4] [6]
2.1 Water: polar covalent bond
CC4: polar covalent bond
e dierences in electronegativity in each of these bonds is greater than 0. [CL 2] (3)
2.2
2 marks for each correct diagram.
-1 any mistake. [CL 3] (4)
2.3 Water: hydrogen bond (dipole-dipole) forces CC
4
: London forces [CL 2] (2)
2.4 e viscosity of water is about 10 times that of CC
4
, and its surface tension is
just a little more than twice as high. e hydrogen bonds between molecules
in water are much stronger than the dispersion (London) forces in CC
4
.
erefore, it makes sense that water molecules will cling to one another more
tightly than CC
4
molecules – and therefore have stronger forces in the surface of
the liquid (surface tension) and greater viscosity than CC
4
. [CL 4] (4)
2.5 Iodine is a non-polar substance, whereas KC is an ionic substance. Substances
with similar strength of intermolecular forces are able to dissolve in one another.
erefore, iodine will dissolve in CC
4
(but KC will not). [CL 3] (3)
[17]
Gr11_Physical_Science_Term1_Resource_Book.indb 121 2018/12/24 8:26:11 AM
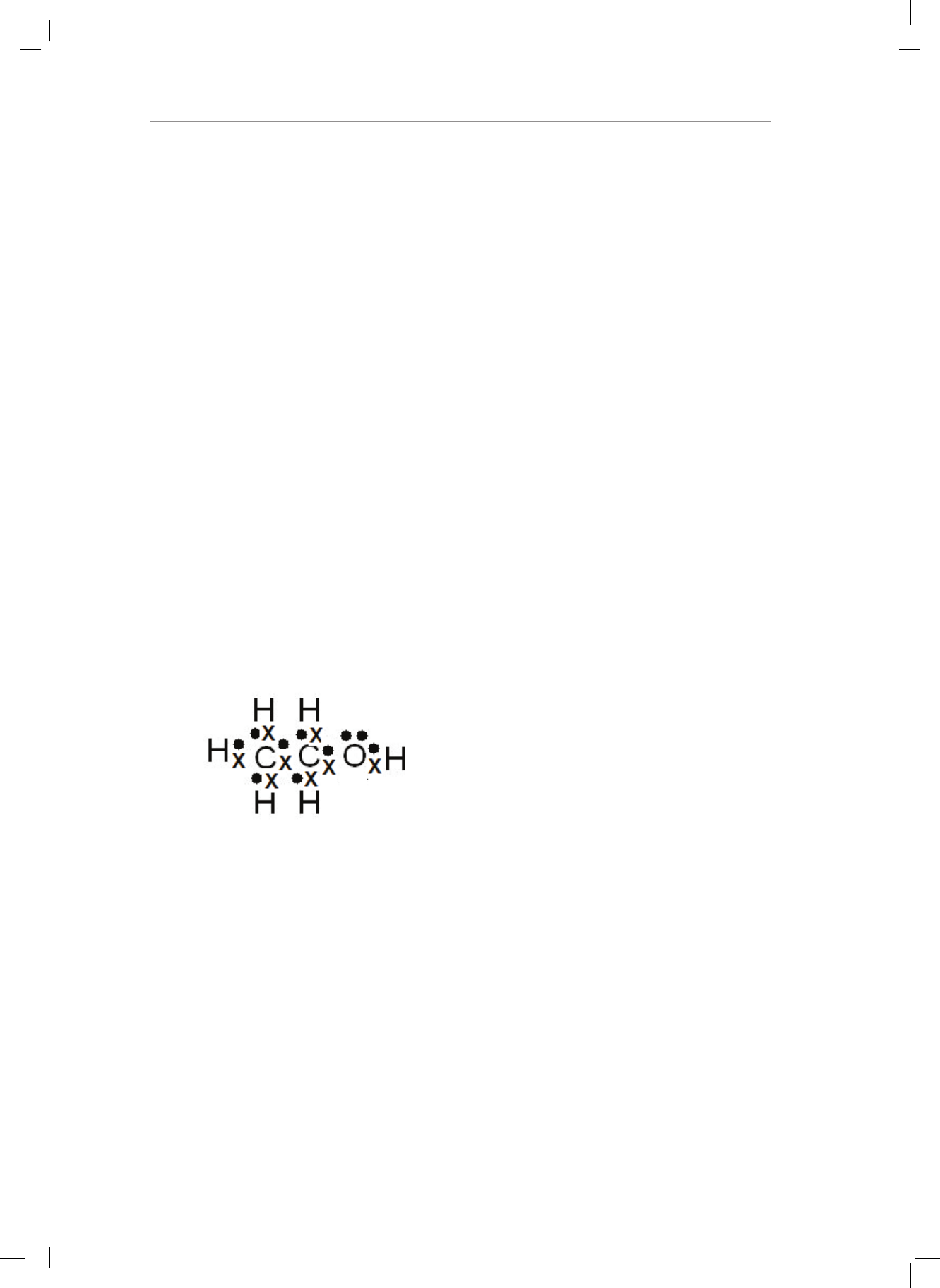
RESOURCE PACK
3.1.1 e melting and boiling points of NH
3
are much higher than those of CF
therefore it takes more energy to overcome the intermolecular forces in NH
3
.
[CL2] (2)
3.1.2 CF
4
molecules are non-polar molecules; they have London forces between them.
Water molecules are polar molecules with stronger hydrogen bonds between
them. If CF
4
is added to water, the water molecules are so strongly attracted to
each other that they will not move apart to allow CF
4
molecules to mix in with
them. erefore CF
4
is insoluble in water.
NH
3
molecules are polar molecules with hydrogen bonding force between them.
Because these forces are of comparable strength to the forces between water
molecules, ammonia molecules will mix with water molecules – ammonia will
dissolve in water. [CL3] (5)
3.1.3 a) NH
3
(g) + H
2
O() NH
4
+
(aq) + OH
−
(aq) [CL3] (1)
b) e lone pair of electrons on the NH
3
molecule is donated to form the bond
with the H
+
ion that is removed from the water molecule. [CL3] (2)
3.2.1 e boiling point of H
2
W is much higher that expected. [CL2] (2)
3.2.2 Water (or H
2
O) [CL2] (2)
3.2.3 Water molecules have much stronger intermolecular forces between their
molecules than the other hydrides of group VI elements do. is occurs
because hydrogen is bonded to oxygen which is a very small atom with high
electronegativity . Hydrogen bonds exist between water molecules; dipole-
dipole forces exist between the molecules of the other substances. [CL3] (4)
[17]
4.1. 4 electrons on each carbon atom
1 electron on each hydrogen atom
6 electrons on oxygen atoms (1 lone pair)
[CL3] (3)
3.1.1 The melting and boiling points of NH
3
are much higher than those of CF
4
therefore it
takes more energy to overcome the intermolecular forces in NH
3
. [CL2] (2)
3.1.2 CF
4
molecules are non-polar molecules; they have London forces between them.
Water molecules are polar molecules with stronger hydrogen bonds between them.
If CF
4
is added to water, the water molecules are so strongly attracted to each
other that they will not move apart to allow CF
4
molecules to mix in with them.
Therefore CF
4
is insoluble in water.
NH
3
molecules are polar molecules with hydrogen bonding force between them.
Because these forces are of comparable strength to the forces between water
molecules, ammonia molecules will mix with water molecules – ammonia will
dissolve in water. [CL3] (5)
3.1.3 a) NH
3
(g) + H
2
O(ℓ) → NH
4
+
(aq) + OH
-
(aq) [CL3] (1)
b) The lone pair of electrons on the NH
3
molecule is donated to form the bond
with the H
+
ion that is removed from the water molecule. [CL3] (2)
3.2.1 The boiling point of H
2
W is much higher that expected. [CL2] (2)
3.2.2 Water (or H
2
O) [CL2] (2)
3.2.3 Water molecules have much stronger intermolecular forces between their molecules
than the other hydrides of group VI elements do. This occurs because hydrogen is
bonded to oxygen which is a very small atom with high electronegativity .
Hydrogen bonds exist between water molecules; dipole-dipole forces exist between
the molecules of the other substances. [CL3] (4)
[17]
4.1.
4 electrons on each carbon atom
1 electron on each hydrogen atom
6 electrons on oxygen atoms (1 lone pair)
[CL3] (3)
4.2 polar covalent bond
The bonded pair of shared electrons is more strongly attracted to the more
electronegative atom (oxygen). [CL2] (3)
4.3 a) tetrahedral [CL2] (1)
b) angular [CL3] (1)
4.4 Ethanol molecules have a non-polar side and a polar side to them therefore they
are able to dissolve polar and non-polar substances. [CL4] (4)
4.2 polar covalent bond
e bonded pair of shared electrons is more strongly attracted to the more
electronegative atom (oxygen). [CL2] (3)
4.3 a) tetrahedral [CL2] (1)
b) angular [CL3] (1)
4.4 Ethanol molecules have a non-polar side and a polar side to them therefore
they are able to dissolve polar and non-polar substances. [CL4] (4)
Gr11_Physical_Science_Term1_Resource_Book.indb 122 2018/12/24 8:26:12 AM
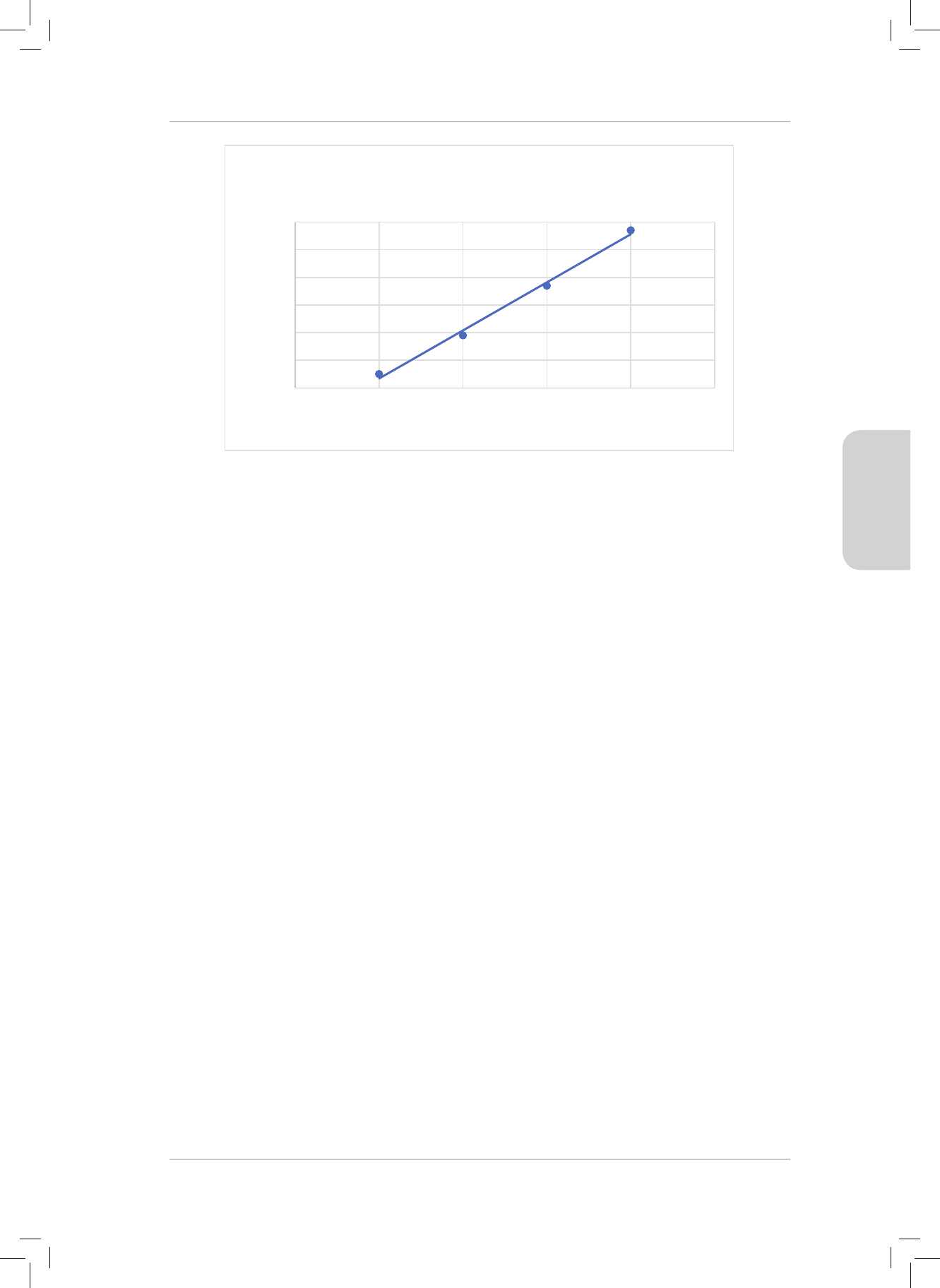
ASSESSMENTS
5.1
5.1
Appropriate title for the graph
Axes labelled correctly with SI unit (y-axis)
Appropriate scales on axes
Points plotted correctly
Line of best fit (straight line) [CL2] (5)
5.2 The boiling point increases at a steady rate as the number of carbon atoms in the
alcohol molecule increases. [CL3] (2)
5.3 Hydrogen bonds [CL2] (1)
5.4 As the number of carbon atoms in the molecules increases, the size of the molecules
also increases. The intermolecular forces between the molecules become stronger
because there are more points of contact available on the bigger molecules.
[CL3] (2)
5.5 Methanol has hydrogen bonding between its molecules whereas ethane which is a
non-polar substance has weaker London forces between its molecules.
Hydrogen bonding forces are the strongest type of intermolecular force; London
forces are the weakest forces between molecules therefore there is a wide
difference between their boiling points. [CL3] (4)
6.1 The boiling point is the temperature at which the vapour pressure of a liquid is equal
to the atmospheric pressure. [CL1] (2)
6.2 NH
3
is a polar molecule which has strong hydrogen bonds between its molecules.
CH
4
is a non-polar molecule with weaker London forces between its molecules. It
takes much more energy to overcome the forces between the molecules of NH
3
therefore its boiling point is much higher than that of CH
4
. [CL3] (3)
60
70
80
90
100
110
120
0 1 2 3 4 5
Boiling point (
o
C)
Number of atoms of carbon in the alcohol molecule
Graph of Boiling Point against Number of Atoms
of Carbon in the Alcohol Molecule
Appropriate title for the graph
Axes labelled correctly with SI unit (y-axis)
Appropriate scales on axes
Points plotted correctly
Line of best t (straight line) [CL2] (5)
5.2 e boiling point increases at a steady rate as the number of carbon atoms in
the alcohol molecule increases. [CL3] (2)
5.3 Hydrogen bonds [CL2] (1)
5.4 As the number of carbon atoms in the molecules increases, the size of the
molecules also increases. e intermolecular forces between the molecules
become stronger because there are more points of contact available on the bigger
molecules. [CL3] (2)
5.5 Methanol has hydrogen bonding between its molecules whereas ethane which
is a non-polar substance has weaker London forces between its molecules.
Hydrogen bonding forces are the strongest type of intermolecular force;
London forces are the weakest forces between molecules therefore there is a
wide dierence between their boiling points. [CL3] (4)
6.1 e boiling point is the temperature at which the vapour pressure of a liquid is
equal to the atmospheric pressure. [CL1] (2)
6.2 NH
3
is a polar molecule which has strong hydrogen bonds between its
molecules. CH
4
is a non-polar molecule with weaker London forces between
its molecules. It takes much more energy to overcome the forces between the
molecules of NH
3
therefore its boiling point is much higher than that of CH
4
.
[CL3] (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 123 2018/12/24 8:26:12 AM

RESOURCE PACK
6.3 NH
3
and H
2
O both have polar molecules with hydrogen bonds between them.
e electronegativity dierence between N and H is 3,0 – 2,1 = 0,9 whereas
the electronegativity dierence between H and O is 3,5 – 2,1 = 1,4. e
covalent bonds inside the water molecule are more polar than those in the NH
3
molecule. Water molecules have an angular shape, whereas NH
3
molecules
are trigonal pyramidal. is allows each water molecule to form two hydrogen
bonds with two other water molecules making the intermolecular forces in water
much stronger than those in NH
3
. [CL4] (4)
6.4.1 NH
3
or H
2
O [CL2] (1)
6.4.2 CH
4
or SiH
4
[CL2] (1)
[14]
7.1 Hydrogen bonds between water molecules act in particular directions. When
ice crystallises, this directional property of hydrogen causes the molecules to
align next to each in a more open structure than they do when water is liquid.
Water’s volume increases as the molecules move into their positions in the
crystal, therefore its density decreases. [CL3] (3)
7.2 Water freezes from the top down so, ice forms on the surface of water rst while
the bottom of the lake, dam, river or ocean remains liquid. e sheet of ice
over the top of a frozen lake insulates the water below it from the colder air in
the atmosphere. In this way plants and animals that live in water are able to do so
under the sheet of ice. [CL2] (2)
7.3 Water has a very high specic heat capacity. (It takes a large amount of energy to
be absorbed per kg of water to raise the temperature of water by 1°C). Water
also releases a large amount of energy per kg when its temperature drops by 1°C.
In this way water acts as a heat reservoir – and regulates the temperatures on
Earth. [CL3] (2)
7.4 Intramolecular forces are stronger than intermolecular forces. In water the
polar covalent bond between hydrogen atoms and oxygen atoms requires more
energy to break, that the energy required to overcome the hydrogen bonding
forces that keep the molecules together in liquids and solids. [CL3] (3)
7.5 Eucalyptus oil molecules are non-polar molecules with weaker London forces
between them. Water molecules have stronger hydrogen bonds between their
molecules. It takes more energy to release a water molecule from the surface
than it does to release molecules of eucalyptus oil. [CL3] (3)
Gr11_Physical_Science_Term1_Resource_Book.indb 124 2018/12/24 8:26:12 AM
